Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines when administered by intradermal route: results of a metaanalysis
- PMID: 20523131
- PMCID: PMC3322518
- DOI: 10.4161/hv.6.7.11934
Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines when administered by intradermal route: results of a metaanalysis
Abstract
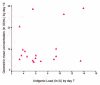
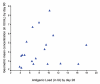
The metadata of 10 published studies and 3 vaccine trial reports comprising of 19 vaccine cohorts from four countries conducted over a period of 23 years (1986 - 2009) was used for metaanalysis. The vaccines studied were purified chick embryo cell vaccine (Rabipur, India & Germany), purified vero cell rabies vaccine (Verorab, France; Indirab, India) & human diploid cell vaccine (MIRV, France).The potency of these vaccines varied from 0.55 IU to 2.32 IU per intradermal dose of 0.1 ml per site. The vaccines were administered to 1,011 subjects comprising of 19 cohorts and using five different ID regimens. The immunogenicity was measured by assays of rabies virus neutralizing antibody (RVNA) titres using rapid fluorescent focus inhibition test (RFFIT) [15 cohorts] and mouse neutralization test (MNT) [4 cohorts]. The statistical analysis of the data was done by Karl Pearson's correlation coefficient to measure the relationship between antigenicity and immunogenicity. It was revealed that, there was no significant linear relationship between antigenicity and immunogenicity of rabies vaccines when administered by intradermal route. (p> 0.230 and p>0.568).
Figures
References
-
- World Health Organization, author. Rabies vaccines, WHO position paper, weekly epidemiological record 49/50. 2007;82:426–426.
-
- Proposed new WHO rabies control guidelines (Highlights from the WHO consultation on human and dog rabies prevention and control, France, Oct. 2009) Asian Biomed. 2009;3:751–754.
-
- Sudarshan MK, Mahendra BJ, Madhusudana SN, Ashwath Narayana DH, Sanjay TV, Gangaboraiah, et al. Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines: Results of a metaanalysis. Hum Vaccin. 2005;1:187–190. - PubMed
-
- Beran J, Honegr K, Banzhoff A, Malerczyk C. Potency requirements of rabies vaccines administered intradermally using the Thai Red Cross regimen: investigation of the immunogenicity of serially diluted purified chick embryo cell rabies vaccine. Vaccine. 2005;23:3902–3927. - PubMed
-
- Lang J, Hoa DQ, Gioi NV, Vien NC, Nguyen CV, Rouyrre N. Immunogenicity and safety of low dose intradermal rabies vaccine given during an Expanded Programme on immunization session in Vietnam: results of a comparative randomized trial. Trans R Soc Trop Med Hyg. 1999;93:208–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources