Gemcitabine combined with gum mastic causes potent growth inhibition and apoptosis of pancreatic cancer cells
- PMID: 20523344
- PMCID: PMC4002976
- DOI: 10.1038/aps.2010.54
Gemcitabine combined with gum mastic causes potent growth inhibition and apoptosis of pancreatic cancer cells
Abstract
Aim: To investigate the antiproliferative and apoptotic effects of gemcitabine combined with gum mastic and the underlying mechanisms in human pancreatic cancer cell lines.
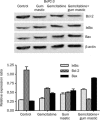
Methods: Cell proliferation and apoptosis were examined using the methyl thiazolyl tetrazolium (MTT) assay and propidium iodine staining, respectively. The expression of Bcl-2, Bax, NF-kappaB p65 subunit, and IkappaBalpha protein was measured using Western blotting.
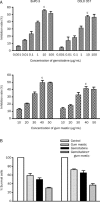
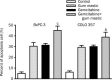
Results: Gemcitabine 0.01-100 microg/mL inhibited cell proliferation and induced apoptosis in both pancreatic cancer BxPC-3 and COLO 357 cells. Gum mastic 40 microg/mL significantly potentiated the antiproliferative and apoptotic effects of gemcitabine 10 microg/mL after 72-h treatment. When cells were treated with gemcitabine in combination with gum mastic, the IkappaBalpha level was increased, whereas NF-kappaB activation was blocked; the expression of Bax protein was substantially increased, but Bcl-2 protein was down-regulated.
Conclusion: Gemcitabine combined with gum mastic causes potent apoptosis in pancreatic cancer cells. The combination may be an effective therapeutic strategy for pancreatic cancer.
Figures

References
-
- Fryer RA, Galustian C, Dalgleish AG. Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol. 2009;4:102–12. - PubMed
-
- Yokoyama Y, Nimura Y, Nagino M. Advances in the treatment of pancreatic cancer: limitations of surgery and evaluation of new therapeutic strategies. Surg Today. 2009;39:466–75. - PubMed
-
- Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642–54. - PubMed
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized-trial. J Clin Oncol. 1997;15:2403–13. - PubMed
-
- Burris HA, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33:18–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

