Mycobacterium tuberculosis transcriptional adaptation, growth arrest and dormancy phenotype development is triggered by vitamin C
- PMID: 20523728
- PMCID: PMC2877710
- DOI: 10.1371/journal.pone.0010860
Mycobacterium tuberculosis transcriptional adaptation, growth arrest and dormancy phenotype development is triggered by vitamin C
Abstract
Background: Tubercle bacilli are thought to persist in a dormant state during latent tuberculosis (TB) infection. Although little is known about the host factors that induce and maintain Mycobacterium tuberculosis (M. tb) within latent lesions, O(2) depletion, nutrient limitation and acidification are some of the stresses implicated in bacterial dormancy development/growth arrest. Adaptation to hypoxia and exposure to NO/CO is implemented through the DevRS/DosT two-component system which induces the dormancy regulon.
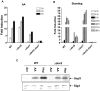
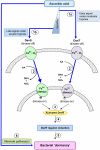
Methodology/principal findings: Here we show that vitamin C (ascorbic acid/AA) can serve as an additional signal to induce the DevR regulon. Physiological levels of AA scavenge O(2) and rapidly induce the DevR regulon at an estimated O(2) saturation of <30%. The kinetics and magnitude of the response suggests an initial involvement of DosT and a sustained DevS-mediated response during bacterial adaptation to increasing hypoxia. In addition to inducing DevR regulon mechanisms, vitamin C induces the expression of selected genes previously shown to be responsive to low pH and oxidative stress, triggers bacterial growth arrest and promotes dormancy phenotype development in M. tb grown in axenic culture and intracellularly in THP-1 cells.
Conclusions/significance: Vitamin C mimics multiple intracellular stresses and has wide-ranging regulatory effects on gene expression and physiology of M. tb which leads to growth arrest and a 'dormant' drug-tolerant phenotype, but in a manner independent of the DevRS/DosT system. The 'AA-dormancy infection model' offers a potential alternative to other models of non-replicating persistence of M. tb and may be useful for investigating host-'dormant' M. tb interactions. Our findings offer a new perspective on the role of nutritional factors in TB and suggest a possible role for vitamin C in TB.
Conflict of interest statement
Figures
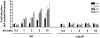
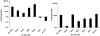

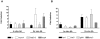

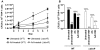
References
-
- Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe. 2007;2:352–364. - PubMed
-
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

