Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice
- PMID: 20523738
- PMCID: PMC2877721
- DOI: 10.1371/journal.pone.0010863
Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice
Abstract
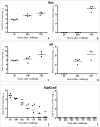
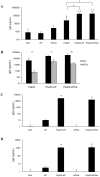
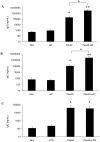
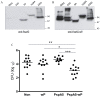
Streptococcus pneumoniae is the leading cause of respiratory acute infections around the world. In Latin America, approximately 20,000 children under 5 years of age die of pneumococcal diseases annually. Pneumococcal surface protein A (PspA) is among the best-characterized pneumococcal antigens that confer protection in animal models of pneumococcal infections and, as such, is a good alternative for the currently available conjugated vaccines. Efficient immune responses directed to PspA in animal models have already been described. Nevertheless, few low cost adjuvants for a subunit pneumococcal vaccine have been proposed to date. Here, we have tested the adjuvant properties of the whole cell Bordetella pertussis vaccine (wP) that is currently part of the DTP (diphtheria-tetanus-pertussis) vaccine administrated to children in several countries, as an adjuvant to PspA. Nasal immunization of BALB/c mice with a combination of PspA5 and wP or wP(low)--a new generation vaccine that contains low levels of B. pertussis LPS--conferred protection against a respiratory lethal challenge with S. pneumoniae. Both PspA5-wP and PspA5-wP(low) vaccines induced high levels of systemic and mucosal antibodies against PspA5, with similar profile, indicating no essential requirement for B. pertussis LPS in the adjuvant properties of wP. Accordingly, nasal immunization of C3H/HeJ mice with PspA5-wP conferred protection against the pneumococcal challenge, thus ruling out a role for TLR4 responses in the adjuvant activity and the protection mechanisms triggered by the vaccines. The high levels of anti-PspA5 antibodies correlated with increased cross-reactivity against PspAs from different clades and also reflected in cross-protection. In addition, passive immunization experiments indicated that antibodies played an important role in protection in this model. Finally, subcutaneous immunization with a combination of PspA5 with DTP(low) protected mice against challenge with two different pneumococcal strains, opening the possibility for the development of a combined infant vaccine composed of DTP and PspA.
Conflict of interest statement
Figures

Similar articles
-
Pertussis toxin improves immune responses to a combined pneumococcal antigen and leads to enhanced protection against Streptococcus pneumoniae.Clin Vaccine Immunol. 2014 Jul;21(7):972-81. doi: 10.1128/CVI.00134-14. Epub 2014 May 7. Clin Vaccine Immunol. 2014. PMID: 24807055 Free PMC article.
-
Pneumococcal Surface Protein A does not affect the immune responses to a combined diphtheria tetanus and pertussis vaccine in mice.Vaccine. 2013 May 7;31(20):2465-70. doi: 10.1016/j.vaccine.2013.03.026. Epub 2013 Mar 26. Vaccine. 2013. PMID: 23541622
-
Protection Elicited by Nasal Immunization with Recombinant Pneumococcal Surface Protein A (rPspA) Adjuvanted with Whole-Cell Pertussis Vaccine (wP) against Co-Colonization of Mice with Streptococcus pneumoniae.PLoS One. 2017 Jan 19;12(1):e0170157. doi: 10.1371/journal.pone.0170157. eCollection 2017. PLoS One. 2017. PMID: 28103277 Free PMC article.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
-
A Jack of All Trades: The Role of Pneumococcal Surface Protein A in the Pathogenesis of Streptococcus pneumoniae.Front Cell Infect Microbiol. 2022 Feb 2;12:826264. doi: 10.3389/fcimb.2022.826264. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186799 Free PMC article. Review.
Cited by
-
Characterization of protective immune responses induced by pneumococcal surface protein A in fusion with pneumolysin derivatives.PLoS One. 2013;8(3):e59605. doi: 10.1371/journal.pone.0059605. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533636 Free PMC article.
-
Streptococcus pneumoniae serotype distribution in low- and middle-income countries of South Asia: Do we need to revisit the pneumococcal vaccine strategy?Hum Vaccin Immunother. 2025 Dec;21(1):2461844. doi: 10.1080/21645515.2025.2461844. Epub 2025 Feb 25. Hum Vaccin Immunother. 2025. PMID: 39999432 Free PMC article. Review.
-
Pertussis toxin improves immune responses to a combined pneumococcal antigen and leads to enhanced protection against Streptococcus pneumoniae.Clin Vaccine Immunol. 2014 Jul;21(7):972-81. doi: 10.1128/CVI.00134-14. Epub 2014 May 7. Clin Vaccine Immunol. 2014. PMID: 24807055 Free PMC article.
-
Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles.Acta Biomater. 2013 Sep;9(9):8262-71. doi: 10.1016/j.actbio.2013.06.006. Epub 2013 Jun 14. Acta Biomater. 2013. PMID: 23774257 Free PMC article.
-
Evaluation of inactivated Bordetella pertussis as a delivery system for the immunization of mice with Pneumococcal Surface Antigen A.PLoS One. 2020 Jan 16;15(1):e0228055. doi: 10.1371/journal.pone.0228055. eCollection 2020. PLoS One. 2020. PMID: 31945121 Free PMC article.
References
-
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
-
- de Quadros CA. From global to regional: the importance of pneumococcal disease in Latin America. Vaccine. 2009;27(Suppl 3):C29–32. - PubMed
-
- Dagan R. New insights on pneumococcal disease: what we have learned over the past decade. Vaccine. 2009;27(Suppl 3):C3–5. - PubMed
-
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

