The proprotein convertase encoded by amontillado (amon) is required in Drosophila corpora cardiaca endocrine cells producing the glucose regulatory hormone AKH
- PMID: 20523747
- PMCID: PMC2877730
- DOI: 10.1371/journal.pgen.1000967
The proprotein convertase encoded by amontillado (amon) is required in Drosophila corpora cardiaca endocrine cells producing the glucose regulatory hormone AKH
Abstract
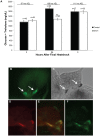
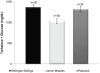
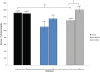
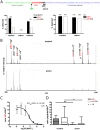
Peptide hormones are potent signaling molecules that coordinate animal physiology, behavior, and development. A key step in activation of these peptide signals is their proteolytic processing from propeptide precursors by a family of proteases, the subtilisin-like proprotein convertases (PCs). Here, we report the functional dissection of amontillado (amon), which encodes the Drosophila homolog of the mammalian PC2 protein, using cell-type specific inactivation and rescue experiments, and we show that amon is required in the islet-like adipokinetic hormone (AKH)-producing cells that regulate sugar homeostasis. In Drosophila, AKH acts analogously to vertebrate glucagon to increase circulating sugar levels from energy stores, while insulin-like peptides (DILPs) act to decrease sugar levels. amon mutant larvae have significantly reduced hemolymph sugar levels, and thus phenocopy larvae where the AKH-producing cells in the corpora cardiaca have been ablated. Reduction of amon expression in these cells via cell-specific RNA inactivation also results in larvae with reduced sugar levels while expression of amon in AKH cells in an amon mutant background rescues hypoglycemia. Hypoglycemia in larvae resulting from amon RNA inactivation in the AKH cells can be rescued by global expression of the akh gene. Finally, mass spectrometric profiling shows that the production of mature AKH is inhibited in amon mutants. Our data indicate that amon function in the AKH cells is necessary to maintain normal sugar homeostasis, that amon functions upstream of akh, and that loss of mature AKH is correlated with loss of amon activity. These observations indicate that the AKH propeptide is a proteolytic target of the amon proprotein convertase and provide evidence for a conserved role of PC2 in processing metabolic peptide hormones.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Sossin WS, Fisher JM, Scheller RH. Cellular and molecular biology of neuropeptide processing and packaging. Neuron. 1989;2:1407–1417. - PubMed
-
- Strand FL. New vistas for melanocortins. Finally, an explanation for their pleiotropic functions. Ann N Y Acad Sci. 1999;897:1–16. - PubMed
-
- Zhou A, Webb G, Zhu XR, Steiner DF. Proteolytic processing in the secretory pathway. Journal of Biological Chemistry. 1999;274:20745–20748. - PubMed
-
- Nillni EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology. 2007;148:4191–4200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

