Catalytic intermolecular hydroamination of vinyl ethers
- PMID: 20523749
- PMCID: PMC2879649
- DOI: 10.1055/s-0029-1218293
Catalytic intermolecular hydroamination of vinyl ethers
Abstract
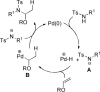
This manuscript details the development of a palladium-catalyzed hydroamination of vinyl ethers. It is proposed that palladium catalyzes the hydroamination via Bronsted base catalysis, where palladium is protonated by the relatively acidic sulfonamide to generate a palladium hydride as well as the active anionic sulfonamide nucleophile. Thus, this process is distinct from known palladium-catalyzed hydroaminations of styrene derivatives that utilize less acidic amines.
References
-
- Mueller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795–3892. - PubMed
- Widenhoefer RA, Han X. Eur. J. Org. Chem. 2006:4555–4563.
- Hazari N, Mountford P. Acc. Chem. Res. 2005;38:839–849. - PubMed
- Hultzsch KC. Org. Biomol. Chem. 2005;3:1819–1824. - PubMed
- Hong S, Marks TJ. Acc. Chem. Res. 2004;37:673–686. - PubMed
- Mueller TE, Beller M. Chem. Rev. 1998;98:675–703. - PubMed
-
- Ohimiya H, Moriya T, Sawamura M. Org. Lett. 2009;11:2145–2147. - PubMed
- Hesp KD, Stradiotto M. Org. Lett. 2009;11:1449–1452. - PubMed
- Biyikal M, Lohnwitz K, Roesky PW, Bechert S. Synlett. 2008:3106–3110.
- Graebe K, Pohlki F, Doye S. Eur. J. Org. Chem. 2008:4815–4823.
- Majumder S, Odom AL. Organometallics. 2008;27:1174–1177.
- Liu Z, Hartwig JF. J. Am. Chem. Soc. 2008;130:1570–1571. - PMC - PubMed
-
-
For recent acid-catalyzed hydroaminations see: Zigang Li, Zhang J, Brouwer C, Yang C-G, Reich NW, He C. Org. Lett. 2006;8:4175–4178.. Motokura K, Nakagiri N, Mori K, Mizugaki T, Ebitani K, Jitsukawa K, Kaneda K. Org. Lett. 2006;8:4617–4620.. Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org. Lett. 2006;8:4179–4182.. Schlummer B, Hartwig JF. Org. Lett. 2002;4:1471–1474.. Colinas PA, Brave RD. Org. Lett. 2003;5:4509–4511.. Toshima K, Nagai H, Ushiki Y, Matsumura S. Synlett. 1998:1007.. Karshtedt D, Bell AT, Tilly TD. J. Am. Chem. Soc. 2005;127:12640–12646.. Widenhoefer R. Angew. Chem., Int. Ed. 2006;45:1747–1749.. Jiao P, Nakashima D, Yamamoto H. Angew. Chem., Int. Ed. 2008;47:2411–2413..
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources