Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line
- PMID: 20523756
- PMCID: PMC2879659
Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line
Abstract
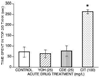
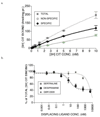
Zebrafish (Danio rerio) associative responses are useful for pharmaceutical and toxicology screening, behavioral genetics, and discovering neural mechanisms involved in behavioral modulation. In novel environments, zebrafish swim to tank bottoms and dark backgrounds, behaviors attributed to anxiety associated with threat of predation. To examine possible genetic effects of inbreeding and segregation on this behavior, we compared Zebrafish International Resource Center (ZIRC) AB and WIK lines to zebrafish and GloFish® from a pet store (PETCO) in two qualitatively different novel environments: the dive tank and aquatic light/dark plus maze. Behavior was observed in the dive tank for 5 min, immediately followed by 5 min in the light/dark plus maze. Among strains, WIK spent more time in the dive tank top than AB (76 ± 30 vs. 17 ± 11 sec), and AB froze in the plus maze center for longer than PETCO or GloFish® (162 ± 61 vs. 72 ± 29 or 27 ± 27 sec). Further, behavior of zebrafish exposed for 3 min to 25 mg/L nicotine, desipramine, chlordiazepoxide, yohimbine, 100 mg/L citalopram, 0.05% DMSO, or 0.5% ethanol was compared to controls. Approximately 0.1% of drug is available in brain after such exposures. Desipramine or citalopram-exposed fish spent more time in the dive tank top, and both reuptake inhibitors bound to serotonin transporters in zebrafish brain with high affinity (K(i) = 7 ± 5 and 9 ± 5 nM). In the plus maze, chlordiazepoxide, ethanol and DMSO-exposed fish crossed more lines and spent more time in white arms. Neither 25 mg/L nicotine nor yohimbine altered zebrafish behavior in novel environments, but nicotine was anxiolytic at higher doses. Overall, the light/dark plus maze and dive tank are distinct behavioral measures that are sensitive to treatment with anxiolytic compounds, but zebrafish line selection and solvents can influence baseline behavior in these tests.
Figures






References
-
- Arthur D, Levin ED. Spatial and non-spatial visual discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131.
-
- Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behavioral Brain Research. 2008;186:107–117. - PubMed
-
- Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behavior Research Methods. 2006;38:456–469. - PubMed
