Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control
- PMID: 20523897
- PMCID: PMC2877741
- DOI: 10.1371/journal.ppat.1000917
Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control
Abstract
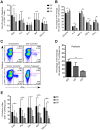
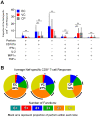
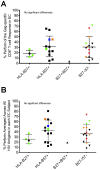
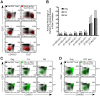
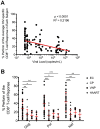
Many immune correlates of CD8(+) T-cell-mediated control of HIV replication, including polyfunctionality, proliferative ability, and inhibitory receptor expression, have been discovered. However, no functional correlates using ex vivo cells have been identified with the known ability to cause the direct elimination of HIV-infected cells. We have recently discovered the ability of human CD8(+) T-cells to rapidly upregulate perforin--an essential molecule for cell-mediated cytotoxicity--following antigen-specific stimulation. Here, we examined perforin expression capability in a large cross-sectional cohort of chronically HIV-infected individuals with varying levels of viral load: elite controllers (n = 35), viremic controllers (n = 29), chronic progressors (n = 27), and viremic nonprogressors (n = 6). Using polychromatic flow cytometry and standard intracellular cytokine staining assays, we measured perforin upregulation, cytokine production, and degranulation following stimulation with overlapping peptide pools encompassing all proteins of HIV. We observed that HIV-specific CD8(+) T-cells from elite controllers consistently display an enhanced ability to express perforin directly ex vivo compared to all other groups. This ability is not restricted to protective HLA-B haplotypes, does not require proliferation or the addition of exogenous factors, is not restored by HAART, and primarily originates from effector CD8(+) T-cells with otherwise limited functional capability. Notably, we found an inverse relationship between HIV-specific perforin expression and viral load. Thus, the capability of HIV-specific CD8(+) T-cells to rapidly express perforin defines a novel correlate of control in HIV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41:1053–1056. - PubMed
-
- Lefrere JJ, Mariotti M, Morand-Joubert L, Thauvin M, Roudot-Thoraval F. Plasma human immunodeficiency virus RNA below 40 Copies/mL is rare in untreated persons even in the first years of infection. J Infect Dis. 1999;180:526–529. - PubMed
-
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. - PubMed
-
- Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol. 2006;7:173–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

