Hsp70 ATPase Modulators as Therapeutics for Alzheimer's and other Neurodegenerative Diseases
- PMID: 20523917
- PMCID: PMC2879647
Hsp70 ATPase Modulators as Therapeutics for Alzheimer's and other Neurodegenerative Diseases
Abstract
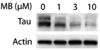
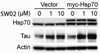
Neurodegenerative diseases caused by abnormal accumulation of the microtubule associated protein tau (MAPT, tau) are collectively called tauopathies. The most devastating tau related disorder is Alzheimer's disease (AD). Molecular chaperones such as heat shock proteins (Hsp) have emerged as critical regulators of tau stability. Several studies from our group and others have shown that the chaperone network can be targeted for the development of therapeutic strategies for AD and other neurodegenerative diseases. Here we will discuss a recent paper and current work from our laboratory where we have manipulated the ATPase activity of the 70-kDa heat shock protein (Hsp70) to regulate tau turnover. A high-throughput screening assay revealed several compounds that activated or inhibited Hsp70's ATPase activity. Inhibitors dramatically and rapidly reduced tau levels, whereas activators stabilized tau, both in cells and brain tissue. Moreover, increased levels of Hsp70 improved ATPase inhibitor efficacy, suggesting that therapies aimed at inducing Hsp70 levels followed by inhibition of its ATPase activity may be a very effective strategy to treat AD. These findings demonstrate that Hsp70 ATPase activity can be targeted to modify the pathologies of AD and other tauopathies.
Conflict of interest statement
No potential conflicts of interest to disclose.
Figures
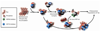
References
-
- Bunker JM, Kamath K, Wilson L, Jordan MA, Feinstein SC. FTDP-17 mutations compromise the ability of tau to regulate microtubule dynamics in cells. J Biol Chem. 2006;281:11856–11863. - PubMed
-
- Trojanowski JQ, Ishihara T, Higuchi M, et al. Amyotrophic lateral sclerosis/parkinsonism dementia complex: transgenic mice provide insights into mechanisms underlying a common tauopathy in an ethnic minority on Guam. Exp Neurol. 2002;176:1–11. - PubMed
-
- Lee VM. Biomedicine. Tauists and beta-aptists united--well almost! Science. 2001;293:1446–1447. - PubMed
-
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
