Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy
- PMID: 20523966
- PMCID: PMC2931643
- DOI: 10.1007/s00125-010-1809-6
Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy
Abstract
Aims/hypothesis: We sought to establish the extent and basis for adaptive changes in beta cell numbers in human pregnancy.
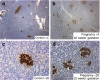
Methods: Pancreas was obtained at autopsy from women who had died while pregnant (n = 18), post-partum (n = 6) or were not pregnant at or shortly before death (controls; n = 20). Pancreases were evaluated for fractional pancreatic beta cell area, islet size and islet fraction of beta cells, beta cell replication (Ki67) and apoptosis (TUNEL), and indirect markers of beta cell neogenesis (insulin-positive cells in ducts and scattered beta cells in pancreas).
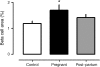
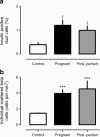
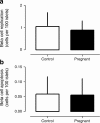
Results: The pancreatic fractional beta cell area was increased by approximately 1.4-fold in human pregnancy, with no change in mean beta cell size. In pregnancy there were more small islets rather than an increase in islet size or beta cells per islet. No increase in beta cell replication or change in beta cell apoptosis was detected, but duct cells positive for insulin and scattered beta cells were increased with pregnancy.
Conclusions/interpretation: The adaptive increase in beta cell numbers in human pregnancy is not as great as in most reports in rodents. This increase in humans is achieved by increased numbers of beta cells in apparently new small islets, rather than duplication of beta cells in existing islets, which is characteristic of pregnancy in rodents.
Figures
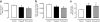
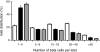
Comment in
-
Beta cell adaptation in pregnancy: a major difference between humans and rodents?Diabetologia. 2010 Oct;53(10):2089-92. doi: 10.1007/s00125-010-1848-z. Epub 2010 Jul 10. Diabetologia. 2010. PMID: 20623217 No abstract available.
References
-
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. - PubMed

