Protein kinase CK2 and new binding partners during spermatogenesis
- PMID: 20524034
- PMCID: PMC11115564
- DOI: 10.1007/s00018-010-0412-9
Protein kinase CK2 and new binding partners during spermatogenesis
Abstract
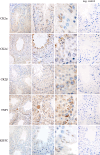
Protein kinase CK2 is an ubiquitously expressed enzyme that is absolutely necessary for the survival of cells. Besides the holoenzyme consisting of the regulatory β-subunit and the catalytic α- or α'-subunit, the subunits exist in separate forms. The subunits bind to a number of other cellular proteins. We show the expression of individual subunits as well as interaction with the transitional nuclear protein TNP1 and with the motor neuron protein KIF5C during spermatogenesis. TNP1 is a newly identified binding partner of the α-subunit of CK2. CK2α and KIF5C were found in late spermatogenesis, whereas CK2β and TNP1 were found in early spermatogenesis. CK2α, CK2α', TNP1, and KIF5C were detected in the acrosome of spermatozoa, while CK2β was detectable in the mid-piece. Combinations of CK2 subunits might determine interactions with other proteins during spermatogenesis. KIF5C as a kinesin motor neuron protein is probably involved in the redistribution of proteins during spermatogenesis.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

