Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases
- PMID: 20524958
- PMCID: PMC2923270
- DOI: 10.1111/j.1471-4159.2010.06834.x
Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases
Abstract
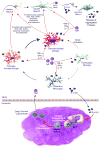
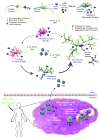
Neurodegenerative diseases, notably Alzheimer's and Parkinson's diseases, are amongst the most devastating disorders afflicting the elderly. Currently, no curative treatments or treatments that interdict disease progression exist. Over the past decade, immunization strategies have been proposed to combat disease progression. Such strategies induce humoral immune responses against misfolded protein aggregates to facilitate their clearance. Robust adaptive immunity against misfolded proteins, however, accelerates disease progression, precipitated by induced effector T cell responses that lead to encephalitis and neuronal death. Since then, mechanisms that attenuate such adaptive neurotoxic immune responses have been sought. We propose that shifting the balance between effector and regulatory T cell activity can attenuate neurotoxic inflammatory events. This review summarizes advances in immune regulation to achieve a homeostatic glial response for therapeutic gain. Promising new ways to optimize immunization schemes and measure their clinical efficacy are also discussed.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
Similar articles
-
Immunization strategies for Parkinson's disease.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1:S218-21. doi: 10.1016/S1353-8020(11)70067-0. Parkinsonism Relat Disord. 2012. PMID: 22166440 Review.
-
Pathways towards an effective immunotherapy for Parkinson's disease.Expert Rev Neurother. 2011 Dec;11(12):1703-15. doi: 10.1586/ern.11.163. Expert Rev Neurother. 2011. PMID: 22091596 Free PMC article. Review.
-
Control of neuroinflammation as a therapeutic strategy for amyotrophic lateral sclerosis and other neurodegenerative disorders.Exp Neurol. 2010 Mar;222(1):1-5. doi: 10.1016/j.expneurol.2009.12.018. Epub 2010 Jan 4. Exp Neurol. 2010. PMID: 20044993 Free PMC article.
-
The innate and adaptive immunological aspects in neurodegenerative diseases.J Neuroimmunol. 2014 Apr 15;269(1-2):1-8. doi: 10.1016/j.jneuroim.2013.09.020. Epub 2013 Oct 5. J Neuroimmunol. 2014. PMID: 24161471 Review.
-
A common vaccine for fighting neurodegenerative disorders: recharging immunity for homeostasis.Trends Pharmacol Sci. 2004 Aug;25(8):407-12. doi: 10.1016/j.tips.2004.06.010. Trends Pharmacol Sci. 2004. PMID: 15276709 Review.
Cited by
-
Granulocyte-macrophage colony-stimulating factor mRNA and Neuroprotective Immunity in Parkinson's disease.Biomaterials. 2021 May;272:120786. doi: 10.1016/j.biomaterials.2021.120786. Epub 2021 Mar 31. Biomaterials. 2021. PMID: 33839625 Free PMC article.
-
Parkinson's disease and systemic inflammation.Parkinsons Dis. 2011 Feb 22;2011:436813. doi: 10.4061/2011/436813. Parkinsons Dis. 2011. PMID: 21403862 Free PMC article.
-
Prospects of statins in Parkinson disease.Neuroscientist. 2011 Jun;17(3):244-55. doi: 10.1177/1073858410385006. Epub 2011 Jan 20. Neuroscientist. 2011. PMID: 21252380 Free PMC article. Review.
-
Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels.Int J Mol Sci. 2018 Feb 24;19(2):640. doi: 10.3390/ijms19020640. Int J Mol Sci. 2018. PMID: 29495280 Free PMC article.
-
Tetracycline repurposing in neurodegeneration: focus on Parkinson's disease.J Neural Transm (Vienna). 2018 Oct;125(10):1403-1415. doi: 10.1007/s00702-018-1913-1. Epub 2018 Aug 14. J Neural Transm (Vienna). 2018. PMID: 30109452 Review.
References
-
- Adams DO, Hamilton TA. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987;97:5–27. - PubMed
-
- Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–1053. - PubMed
-
- Ajmone-Cat MA, Nicolini A, Minghetti L. Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem. 2003;87:1193–1203. - PubMed
-
- Alafuzoff I, Overmyer M, Helisalmi S, Soininen H. Lower counts of astroglia and activated microglia in patients with Alzheimer's disease with regular use of non-steroidal anti-inflammatory drugs. J Alzheimers Dis. 2000;2:37–46. - PubMed
-
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- 2R37NS36126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01NS43985/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- 5P01NS31492/NS/NINDS NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- U54 NS043011/NS/NINDS NIH HHS/United States
- P01 DA026146/DA/NIDA NIH HHS/United States
- 2R01NS034239/NS/NINDS NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R21 NS049246/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- P20RR15635/RR/NCRR NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 NS036127/NS/NINDS NIH HHS/United States
- T32 NS007488/NS/NINDS NIH HHS/United States
- R01 NS070190/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

