Alternative splicing expands the repertoire of dominant JAZ repressors of jasmonate signaling
- PMID: 20525008
- PMCID: PMC2966510
- DOI: 10.1111/j.1365-313X.2010.04265.x
Alternative splicing expands the repertoire of dominant JAZ repressors of jasmonate signaling
Abstract
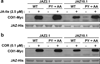
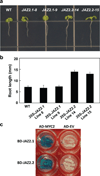
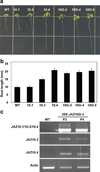
Jasmonates (JAs) are fatty acid-derived signaling compounds that control diverse aspects of plant growth, development and immunity. The F-box protein COI1 functions both as a receptor for jasmonoyl-l-isoleucine (JA-Ile) and as the component of an E3-ubiquitin ligase complex (SCF(COI1) ) that targets JAZ transcriptional regulators for degradation. A key feature of JAZ proteins is the C-terminal Jas motif that mediates the JA-Ile-dependent interaction with COI1. Here, we show that most JAZ genes from evolutionarily diverse plants contain a conserved intron that splits the Jas motif into 20 N-terminal and seven C-terminal (X(5) PY) amino acid submotifs. In most members of the Arabidopsis JAZ family, alternative splicing events involving retention of this intron generate proteins that are truncated before the X(5) PY sequence. In vitro pull-down and yeast two-hybrid assays indicate that these splice variants have reduced capacity to form stable complexes with COI1 in the presence of the bioactive stereoisomer of the hormone (3R,7S)-JA-Ile. cDNA overexpression studies showed that some, but not all, truncated splice variants are dominant repressors of JA signaling. We also show that strong constitutive expression of an intron-containing JAZ10 genomic clone is sufficient to repress JA responses. These findings provide evidence for functional differences between JAZ isoforms, and establish a direct link between the alternative splicing of JAZ pre-mRNA and the dominant repression of JA signal output. We propose that production of dominant JAZ repressors by alternative splicing reduces the negative consequences associated with inappropriate or hyperactivation of the JA response pathway.
© 2010 The Authors. Journal compilation © 2010 Blackwell Publishing Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

