Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period
- PMID: 20525087
- PMCID: PMC2871994
- DOI: 10.1111/j.1469-7580.2010.01219.x
Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period
Abstract
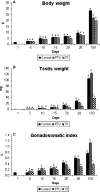
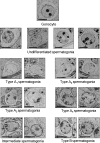
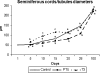
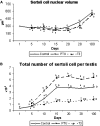
The role of thyroid hormones in testis structure and function has been fairly well studied in laboratory rodents. However, there are no comprehensive data in the literature for mice regarding the effects of transiently induced neonatal hypo- and hyperthyroidism on testis and spermatogonial cell development from birth to adulthood. Our goals were to evaluate the effects of propylthiouracil (PTU) and triidothyronine (T3) on Sertoli cell proliferation/differentiation and to correlate these events with the evolution of the spermatogenic process, tubular lumen formation, blood vessel volume density, and size and number of different spermatogonial types. Although Sertoli cell maturation was accelerated or delayed, respectively, in T3- and PTU-treated mice, the pace of the germ cell maturation was only slightly altered before puberty and the period of Sertoli cell proliferation was apparently not affected by the treatments. However, compared with controls, the total number of Sertoli cells per testis from 10 days of age to adulthood was significantly increased and decreased in PTU- and T3-treated mice, respectively. In comparison to all other spermatogonia, type A(2) was the largest cell in all ages and groups investigated. The PTU-treated mice had a significantly increased total number of undifferentiated spermatogonia as well as volume and percentage of vessels/capillaries, probably due to the higher number of Sertoli cells, particularly at 10 days of age. Taken together, our results suggest that neonatal hypothyroidism may be a valuable tool for studying spermatogonial biology as well as a means for providing more spermatogonial stem cells that could potentially be used for spermatogonial transplantation, thereby optimizing the efficiency of this technique when young mice are used as donors.
Figures





Similar articles
-
Sertoli cells dictate spermatogonial stem cell niches in the mouse testis.Biol Reprod. 2011 Apr;84(4):639-45. doi: 10.1095/biolreprod.110.087320. Epub 2010 Nov 17. Biol Reprod. 2011. PMID: 21084712 Free PMC article.
-
Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment.Biol Reprod. 1994 Nov;51(5):1000-5. doi: 10.1095/biolreprod51.5.1000. Biol Reprod. 1994. PMID: 7531505
-
Characterizing the Spermatogonial Response to Retinoic Acid During the Onset of Spermatogenesis and Following Synchronization in the Neonatal Mouse Testis.Biol Reprod. 2016 Oct;95(4):81. doi: 10.1095/biolreprod.116.141770. Epub 2016 Aug 3. Biol Reprod. 2016. PMID: 27488029 Free PMC article.
-
Testis structure, spermatogonial niche and Sertoli cell efficiency in Neotropical fish.Gen Comp Endocrinol. 2019 Mar 1;273:218-226. doi: 10.1016/j.ygcen.2018.09.004. Epub 2018 Sep 5. Gen Comp Endocrinol. 2019. PMID: 30195025 Review.
-
Comparing the adult and pre-pubertal testis: Metabolic transitions and the change in the spermatogonial stem cell metabolic microenvironment.Andrology. 2023 Sep;11(6):1132-1146. doi: 10.1111/andr.13397. Epub 2023 Feb 3. Andrology. 2023. PMID: 36690000 Free PMC article. Review.
Cited by
-
Thyroid hormone action in the developing testis: intergenerational epigenetics.J Endocrinol. 2020 Feb 17;244(3):R33-R46. doi: 10.1530/JOE-19-0550. J Endocrinol. 2020. PMID: 31977317 Free PMC article. Review.
-
Spermatogonial stem cell markers and niche in equids.PLoS One. 2012;7(8):e44091. doi: 10.1371/journal.pone.0044091. Epub 2012 Aug 28. PLoS One. 2012. PMID: 22937157 Free PMC article.
-
FBXO38 Ubiquitin Ligase Controls Sertoli Cell Maturation.Front Cell Dev Biol. 2022 Jun 13;10:914053. doi: 10.3389/fcell.2022.914053. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35769260 Free PMC article.
-
Targeted disruption of galectin 3 in mice delays the first wave of spermatogenesis and increases germ cell apoptosis.Cell Mol Life Sci. 2021 Apr;78(7):3621-3635. doi: 10.1007/s00018-021-03757-2. Epub 2021 Jan 28. Cell Mol Life Sci. 2021. PMID: 33507326 Free PMC article.
-
Sertoli cells dictate spermatogonial stem cell niches in the mouse testis.Biol Reprod. 2011 Apr;84(4):639-45. doi: 10.1095/biolreprod.110.087320. Epub 2010 Nov 17. Biol Reprod. 2011. PMID: 21084712 Free PMC article.
References
-
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:238–248. - PubMed
-
- Amann RP, Almquist JO. Reproductive capacity of dairy bulls. Direct and indirect measurement of testicular sperm production. J Dairy Sci. 1962;45:774–781.
-
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. - PubMed
-
- Chiarini-Garcia H, Russell LD. High resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–1178. - PubMed
-
- Chiarini-Garcia H, Russell LD. Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction. 2002;123:567–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

