VEGF and its role in the early development of the long bone epiphysis
- PMID: 20525089
- PMCID: PMC2871997
- DOI: 10.1111/j.1469-7580.2010.01223.x
VEGF and its role in the early development of the long bone epiphysis
Abstract
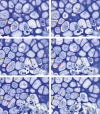
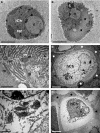
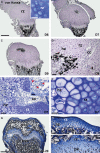
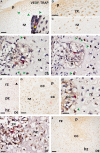
In long bones of murine species, undisturbed development of the epiphysis depends on the generation of vascularized cartilage canals shortly after birth. Despite its importance, it is still under discussion how this event is exactly regulated. It was suggested previously that, following increased hypoxia in the epiphyseal core, angiogenic factors are expressed and hence stimulate the ingrowth of the vascularized canals. In the present study, we tested this model and examined the spatio-temporal distribution of two angiogenic molecules during early development in mice. In addition, we investigated the onset of cartilage hypertrophy and mineralization. Our results provide evidence that the vascular endothelial growth factor is expressed in the epiphyseal resting cartilage prior to the moment of canal formation and is continuously expressed until the establishment of a large secondary ossification centre. Interestingly, we found no expression of secretoneurin before the establishment of the canals although this factor attracts blood vessels under hypoxic conditions. Epiphyseal development further involves maturation of the resting chondrocytes into hypertrophic ones, associated with the mineralization of the cartilage matrix and eventual death of the latter cells. Our results suggest that vascular endothelial growth factor is the critical molecule for the generation of the epiphyseal vascular network in mice long bones. Secretoneurin, however, does not appear to be a player in this event. Hypertrophic chondrocytes undergo cell death by a mechanism interpreted as chondroptosis.
Figures



Similar articles
-
Basement membrane composition of cartilage canals during development and ossification of the epiphysis.Anat Rec. 1995 Mar;241(3):425-37. doi: 10.1002/ar.1092410318. Anat Rec. 1995. PMID: 7755183
-
A mathematical model of epiphyseal development: hypothesis on the cartilage canals growth.Comput Methods Biomech Biomed Engin. 2010 Dec;13(6):765-72. doi: 10.1080/10255841003606116. Comput Methods Biomech Biomed Engin. 2010. PMID: 20526919
-
Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes.Dev Dyn. 2007 Aug;236(8):2077-88. doi: 10.1002/dvdy.21228. Dev Dyn. 2007. PMID: 17626280
-
Structure, formation and role of cartilage canals in the developing bone.Ann Anat. 2008;190(4):305-15. doi: 10.1016/j.aanat.2008.02.004. Epub 2008 May 28. Ann Anat. 2008. PMID: 18602255 Review.
-
Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development.J Cell Biochem. 1996 Jul;62(1):1-9. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. J Cell Biochem. 1996. PMID: 8836870 Review.
Cited by
-
IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation During Postnatal Bone Development.J Bone Miner Res. 2015 Dec;30(12):2239-48. doi: 10.1002/jbmr.2563. Epub 2015 Jul 29. J Bone Miner Res. 2015. PMID: 26011431 Free PMC article.
-
Development of articular cartilage and the metaphyseal growth plate: the localization of TRAP cells, VEGF, and endostatin.J Anat. 2011 Jun;218(6):608-18. doi: 10.1111/j.1469-7580.2011.01377.x. Epub 2011 Apr 3. J Anat. 2011. PMID: 21457260 Free PMC article.
-
Role of tartrate-resistant acid phosphatase (TRAP) in long bone development.Mech Dev. 2012 Jul;129(5-8):162-76. doi: 10.1016/j.mod.2012.04.003. Epub 2012 May 8. Mech Dev. 2012. PMID: 22579636 Free PMC article.
-
Growing Pains: The Need for Engineered Platforms to Study Growth Plate Biology.Adv Healthc Mater. 2022 Oct;11(19):e2200471. doi: 10.1002/adhm.202200471. Epub 2022 Aug 15. Adv Healthc Mater. 2022. PMID: 35905390 Free PMC article. Review.
-
Periarticular Mesenchymal Progenitors Initiate and Contribute to Secondary Ossification Center Formation During Mouse Long Bone Development.Stem Cells. 2019 May;37(5):677-689. doi: 10.1002/stem.2975. Epub 2019 Feb 6. Stem Cells. 2019. PMID: 30681752 Free PMC article.
References
-
- Álvarez J, Costales L, Serra R, et al. Expression patterns of matrix metalloproteinases and vascular endothelial growth factor during epiphyseal ossification. J Bone Miner Res. 2005a;20:1011–1021. - PubMed
-
- Álvarez J, Costales L, López-Muñiz A, et al. Chondrocytes are released as viable cells during cartilage resorption associated with the formation of intrachondral canals in the rat tibial epiphysis. Cell Tissue Res. 2005b;320:501–507. - PubMed
-
- Aszódi A, Bateman JF, Gustafsson E, et al. Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice. Cell Struct Funct. 2000;25:73–84. - PubMed
-
- Blumer MJF, Gahleitner P, Narzt T, et al. Ribbons of semithin sections: an advanced method with a new type of diamond knife. J Neurosci Methods. 2002;120:11–16. - PubMed
-
- Blumer MJF, Longato S, Schwarzer C, et al. Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes. Dev Dyn. 2007;236:2077–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

