Mechanisms of degranulation in neutrophils
- PMID: 20525154
- PMCID: PMC2876182
- DOI: 10.1186/1710-1492-2-3-98
Mechanisms of degranulation in neutrophils
Abstract
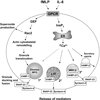
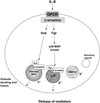
Neutrophils are critical inflammatory cells that cause tissue damage in a range of diseases and disorders. Being bone marrow-derived white blood cells, they migrate from the bloodstream to sites of tissue inflammation in response to chemotactic signals and induce inflammation by undergoing receptor-mediated respiratory burst and degranulation. Degranulation from neutrophils has been implicated as a major causative factor in pulmonary disorders, including severe asphyxic episodes of asthma. However, the mechanisms that control neutrophil degranulation are not well understood. Recent observations indicate that granule release from neutrophils depends on activation of intracellular signalling pathways, including beta-arrestins, the Rho guanosine triphosphatase Rac2, soluble NSF attachment protein (SNAP) receptors, the src family of tyrosine kinases, and the tyrosine phosphatase MEG2. Some of these observations suggest that degranulation from neutrophils is selective and depends on nonredundant signalling pathways. This review focuses on new findings from the literature on the mechanisms that control the release of granule-derived mediators from neutrophils.
Figures
References
-
- Skubitz KM. In: Wintrobe's clinical hematology. Lee GR, Foerster J, Lukens J, et al, editor. Vol. 1. Baltimore: Williams & Wilkins; 1999. Neutrophilic leukocytes; pp. 300–50.
-
- Brinkmann V, Reichard U, Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. - PubMed
-
- Martinelli S, Urosevic M, Daryadel A. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279:44123–32. - PubMed
-
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. - PubMed
-
- Kjeldsen L, Sengelov H, Lollike K. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83:1640–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

