Neutralization of X4- and R5-tropic HIV-1 NL4-3 variants by HOCl-modified serum albumins
- PMID: 20525179
- PMCID: PMC2887905
- DOI: 10.1186/1756-0500-3-155
Neutralization of X4- and R5-tropic HIV-1 NL4-3 variants by HOCl-modified serum albumins
Abstract
Background: Myeloperoxidase (MPO), an important element of the microbicidal activity of neutrophils, generates hypochlorous acid (HOCl) from H2O2 and chloride, which is released into body fluids. Besides its direct microbicidal activity, HOCl can react with amino acid residues and HOCl-modified proteins can be detected in vivo.
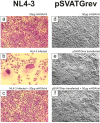
Findings: This report is based on binding studies of HOCl-modified serum albumins to HIV-1 gp120 and three different neutralization assays using infectious virus. The binding studies were carried out by surface plasmon resonance spectroscopy and by standard ELISA techniques. Virus neutralization assays were carried out using HIV-1 NL4-3 virus and recombinant strains with CXCR4 and CCR5 coreceptor usage. Viral infection was monitored by a standard p24 or X-gal staining assay. Our data demonstrate that HOCl-modified mouse-, bovine- and human serum albumins all bind to the HIV-1 NL4-3 gp120 (LAV) glycoprotein in contrast to non-modified albumin. Binding of HOCl-modified albumin to gp120 correlated to the blockade of CD4 as well as that of V3 loop specific monoclonal antibody binding. In neutralization experiments, HOCl-modified serum albumins inhibited replication and syncytium formation of the X4- and R5-tropic NL4-3 isolates in a dose dependent manner.
Conclusions: Our data indicate that HOCl-modified serum albumin veils the binding site for CD4 and the V3 loop on gp120. Such masking of the viral gp120/gp41 envelope complex might be a simple but promising strategy to inactivate HIV-1 and therefore prevent infection when HOCl-modified serum albumin is applied, for example, as a topical microbicide.
Figures





Similar articles
-
HIV-1 dual/mixed tropic isolates show different genetic and phenotypic characteristics and response to maraviroc in vitro.Antiviral Res. 2011 Apr;90(1):42-53. doi: 10.1016/j.antiviral.2011.02.005. Epub 2011 Feb 22. Antiviral Res. 2011. PMID: 21349294
-
The N-linked glycan g15 within the V3 loop of the HIV-1 external glycoprotein gp120 affects coreceptor usage, cellular tropism, and neutralization.Virology. 2002 Dec 5;304(1):70-80. doi: 10.1006/viro.2002.1760. Virology. 2002. PMID: 12490404
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
Mutations That Increase the Stability of the Postfusion gp41 Conformation of the HIV-1 Envelope Glycoprotein Are Selected by both an X4 and R5 HIV-1 Virus To Escape Fusion Inhibitors Corresponding to Heptad Repeat 1 of gp41, but the gp120 Adaptive Mutations Differ between the Two Viruses.J Virol. 2019 May 15;93(11):e00142-19. doi: 10.1128/JVI.00142-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894471 Free PMC article.
-
Shift of clinical human immunodeficiency virus type 1 isolates from X4 to R5 and prevention of emergence of the syncytium-inducing phenotype by blockade of CXCR4.J Virol. 1999 Jul;73(7):5577-85. doi: 10.1128/JVI.73.7.5577-5585.1999. J Virol. 1999. PMID: 10364306 Free PMC article.
Cited by
-
SRSF1 acts as an IFN-I-regulated cellular dependency factor decisively affecting HIV-1 post-integration steps.Front Immunol. 2022 Nov 15;13:935800. doi: 10.3389/fimmu.2022.935800. eCollection 2022. Front Immunol. 2022. PMID: 36458014 Free PMC article.
-
Mucosal immunity in the female genital tract, HIV/AIDS.Biomed Res Int. 2014;2014:350195. doi: 10.1155/2014/350195. Epub 2014 Sep 15. Biomed Res Int. 2014. PMID: 25313360 Free PMC article. Review.
-
Transduction Efficiency of Zika Virus E Protein Pseudotyped HIV-1gfp and Its Oncolytic Activity Tested in Primary Glioblastoma Cell Cultures.Cancers (Basel). 2024 Feb 17;16(4):814. doi: 10.3390/cancers16040814. Cancers (Basel). 2024. PMID: 38398205 Free PMC article.
-
Effect of lysine to arginine mutagenesis in the V3 loop of HIV-1 gp120 on viral entry efficiency and neutralization.PLoS One. 2015 Mar 18;10(3):e0119879. doi: 10.1371/journal.pone.0119879. eCollection 2015. PLoS One. 2015. PMID: 25785610 Free PMC article.
-
Development of Zika Virus E Variants for Pseudotyping Retroviral Vectors Targeting Glioblastoma Cells.Int J Mol Sci. 2023 Sep 23;24(19):14487. doi: 10.3390/ijms241914487. Int J Mol Sci. 2023. PMID: 37833934 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

