Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis
- PMID: 20525198
- PMCID: PMC2911898
- DOI: 10.1186/ar3041
Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis
Abstract
Introduction: Type 4 phosphodiesterases (PDE4) play an important role in immune cells through the hydrolysis of the second messenger, cAMP. Inhibition of PDE4 has previously been shown to suppress immune and inflammatory responses, demonstrating PDE4 to be a valid therapeutic target for immune-mediated pathologies. We assessed the anti-inflammatory effects of a novel PDE4 inhibitor, apremilast, in human synovial cells from rheumatoid arthritis (RA) patients, as well as two murine models of arthritis.
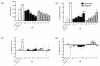
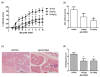
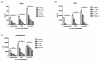
Methods: Cells liberated from tissue excised from arthritic joints of RA patients were cultured in the presence of increasing concentrations of apremilast for 48 hours and spontaneous tumour necrosis factor-alpha (TNFalpha) production was analysed in culture supernatants by ELISA. In addition, arthritis was induced in BALB/c and DBA/1 mice by passive transfer of anti-type II collagen mAb and immunisation with type II collagen, respectively. Mice with established arthritis received 5 or 25 mg/kg apremilast and disease severity was monitored relative to mice receiving vehicle alone. At the end of the study, paws were removed and processed for histopathological assessment. Behavioural effects of apremilast, relative to rolipram, were assessed in naïve DBA/1 mice using an automated activity monitor (LABORAS).
Results: Apremilast dose dependently inhibited spontaneous release of TNFalpha from human rheumatoid synovial membrane cultures. Furthermore, apremilast significantly reduced clinical score in both murine models of arthritis over a ten day treatment period and maintained a healthy joint architecture in a dose-dependent manner. Importantly, unlike rolipram, apremilast demonstrated no adverse behavioural effects in naïve mice.
Conclusions: Apremilast is an orally available PDE4 inhibitor that reduces TNFalpha production from human synovial cells and significantly suppresses experimental arthritis. Apremilast appears to be a potential new agent for the treatment of rheumatoid arthritis.
Figures

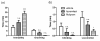
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

