A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats
- PMID: 20525213
- PMCID: PMC2887800
- DOI: 10.1186/1743-422X-7-114
A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats
Abstract
Background: Respiratory syncytial virus (RSV) is a primary cause of serious lower respiratory tract illness for which there is still no safe and effective vaccine available. Using reverse genetics, recombinant (r)RSV and an rRSV lacking the G gene (DeltaG) were constructed based on a clinical RSV isolate (strain 98-25147-X).
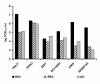
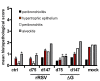
Results: Growth of both recombinant viruses was equivalent to that of wild type virus in Vero cells, but was reduced in human epithelial cells like Hep-2. Replication in cotton rat lungs could not be detected for DeltaG, while rRSV was 100-fold attenuated compared to wild type virus. Upon single dose intranasal administration in cotton rats, both recombinant viruses developed high levels of neutralizing antibodies and conferred comparable long-lasting protection against RSV challenge; protection against replication in the lungs lasted at least 147 days and protection against pulmonary inflammation lasted at least 75 days.
Conclusion: Collectively, the data indicate that a single dose immunization with the highly attenuated DeltaG as well as the attenuated rRSV conferred long term protection in the cotton rat against subsequent RSV challenge, without inducing vaccine enhanced pathology. Since DeltaG is not likely to revert to a less attenuated phenotype, we plan to evaluate this deletion mutant further and to investigate its potential as a vaccine candidate against RSV infection.
Figures


Similar articles
-
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge.J Virol. 2017 May 12;91(11):e00066-17. doi: 10.1128/JVI.00066-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28298602 Free PMC article.
-
A Recombinant Respiratory Syncytial Virus Vaccine Candidate Attenuated by a Low-Fusion F Protein Is Immunogenic and Protective against Challenge in Cotton Rats.J Virol. 2016 Jul 27;90(16):7508-7518. doi: 10.1128/JVI.00012-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27279612 Free PMC article.
-
A Novel Live Attenuated Respiratory Syncytial Virus Vaccine Candidate with Mutations in the L Protein SAM Binding Site and the G Protein Cleavage Site Is Protective in Cotton Rats and a Rhesus Macaque.J Virol. 2021 Jan 13;95(3):e01568-20. doi: 10.1128/JVI.01568-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177201 Free PMC article.
-
Evaluation of the Safety and Immune Efficacy of Recombinant Human Respiratory Syncytial Virus Strain Long Live Attenuated Vaccine Candidates.Virol Sin. 2021 Aug;36(4):706-720. doi: 10.1007/s12250-021-00345-3. Epub 2021 Feb 9. Virol Sin. 2021. PMID: 33559831 Free PMC article. Review.
-
RI-002, an intravenous immunoglobulin containing high titer neutralizing antibody to RSV and other respiratory viruses for use in primary immunodeficiency disease and other immune compromised populations.Expert Rev Clin Immunol. 2017 Dec;13(12):1107-1119. doi: 10.1080/1744666X.2017.1389647. Epub 2017 Oct 16. Expert Rev Clin Immunol. 2017. PMID: 29035131 Free PMC article. Review.
Cited by
-
Immunological Features of Respiratory Syncytial Virus-Caused Pneumonia-Implications for Vaccine Design.Int J Mol Sci. 2017 Mar 4;18(3):556. doi: 10.3390/ijms18030556. Int J Mol Sci. 2017. PMID: 28273842 Free PMC article. Review.
-
Breaking in: human metapneumovirus fusion and entry.Viruses. 2013 Jan 16;5(1):192-210. doi: 10.3390/v5010192. Viruses. 2013. PMID: 23325326 Free PMC article. Review.
-
Respiratory Syncytial Virus Infects Primary Neonatal and Adult Natural Killer Cells and Affects Their Antiviral Effector Function.J Infect Dis. 2019 Feb 15;219(5):723-733. doi: 10.1093/infdis/jiy566. J Infect Dis. 2019. PMID: 30252097 Free PMC article.
-
Will Attention by Vaccine Developers to the Host's Nuclear Hormone Levels and Immunocompetence Improve Vaccine Success?Vaccines (Basel). 2019 Feb 27;7(1):26. doi: 10.3390/vaccines7010026. Vaccines (Basel). 2019. PMID: 30818795 Free PMC article. Review.
-
Respiratory Syncytial Virus Attachment Glycoprotein Contribution to Infection Depends on the Specific Fusion Protein.J Virol. 2015 Oct 14;90(1):245-53. doi: 10.1128/JVI.02140-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468535 Free PMC article.
References
-
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

