Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis
- PMID: 20525214
- PMCID: PMC2891637
- DOI: 10.1186/1471-2407-10-252
Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis
Abstract
Background: The combination of bevacizumab and irinotecan is a new chemotherapy protocol increasingly used for recurrent malignant glioma. Results from phase II trials suggest this drug combination is beneficial to patients, but no conclusive comparisons between this and other treatment protocols have been published.
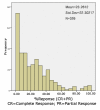
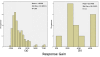
Methods: We performed a systematic review and survival gain analysis of phase II studies to evaluate the efficacy and safety of bevacizumab plus irinotecan treatment. To do this, we utilized a preexisting database from which the mean overall survival and response rate of patients could be predicted. Survival gain, which characterized the influence of treatment, was defined as the difference between observed and predicted mean overall survival. Response gain was calculated similarly.
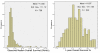
Results: 741 cohorts were enrolled in the database. Among them, 282 cohorts were based on recurrent adult HGG, mean reported median overall survival was 10.96 +/- 8.4 months, and mean response rate was 18.9% +/- 20.5. We found that compared with other treatment protocols, bevacizumab plus irinotecan largely improved response rates (P = 0.00002) and had a possible moderate effect on overall survival time (P = 0.024). Hemorrhage, thromboembolic complications, and gastrointestinal toxicities were the most frequently reported side effects.
Conclusion: The combination of bevacizumab and irinotecan might improve outcome in patients with recurrent malignant glioma. Randomized controlled trials are recommended to evaluate this treatment protocol and the additional value of irinotecan.
Figures

Similar articles
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
Procarbazine, lomustine and vincristine for recurrent high-grade glioma.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD011773. doi: 10.1002/14651858.CD011773.pub2. Cochrane Database Syst Rev. 2017. PMID: 28744879 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
Cited by
-
Phospholipid metabolites in recurrent glioblastoma: in vivo markers detect different tumor phenotypes before and under antiangiogenic therapy.PLoS One. 2013;8(3):e56439. doi: 10.1371/journal.pone.0056439. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520454 Free PMC article. Clinical Trial.
-
Comparing [18F]FET PET and [18F]FDOPA PET for glioma recurrence diagnosis: a systematic review and meta-analysis.Front Oncol. 2024 Jan 10;13:1346951. doi: 10.3389/fonc.2023.1346951. eCollection 2023. Front Oncol. 2024. PMID: 38269019 Free PMC article.
-
Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update.Int J Mol Sci. 2021 May 5;22(9):4899. doi: 10.3390/ijms22094899. Int J Mol Sci. 2021. PMID: 34063168 Free PMC article. Review.
-
Localized targeted antiangiogenic drug delivery for glioblastoma.J Neurooncol. 2018 Apr;137(2):223-231. doi: 10.1007/s11060-018-2747-2. Epub 2018 Jan 11. J Neurooncol. 2018. PMID: 29327174 Review.
-
Bevacizumab plus irinotecan in recurrent or progressive malign glioma: a multicenter study of the Anatolian Society of Medical Oncology (ASMO).J Cancer Res Clin Oncol. 2013 May;139(5):829-35. doi: 10.1007/s00432-013-1390-8. Epub 2013 Feb 12. J Cancer Res Clin Oncol. 2013. PMID: 23400732 Free PMC article.
References
-
- Stupp R, Mason WP, den Bent MJ v, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–93. doi: 10.1054/bjoc.2000.1316. - DOI - PMC - PubMed
-
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

