Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis
- PMID: 20525214
- PMCID: PMC2891637
- DOI: 10.1186/1471-2407-10-252
Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis
Abstract
Background: The combination of bevacizumab and irinotecan is a new chemotherapy protocol increasingly used for recurrent malignant glioma. Results from phase II trials suggest this drug combination is beneficial to patients, but no conclusive comparisons between this and other treatment protocols have been published.
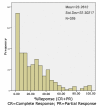
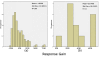
Methods: We performed a systematic review and survival gain analysis of phase II studies to evaluate the efficacy and safety of bevacizumab plus irinotecan treatment. To do this, we utilized a preexisting database from which the mean overall survival and response rate of patients could be predicted. Survival gain, which characterized the influence of treatment, was defined as the difference between observed and predicted mean overall survival. Response gain was calculated similarly.
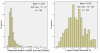
Results: 741 cohorts were enrolled in the database. Among them, 282 cohorts were based on recurrent adult HGG, mean reported median overall survival was 10.96 +/- 8.4 months, and mean response rate was 18.9% +/- 20.5. We found that compared with other treatment protocols, bevacizumab plus irinotecan largely improved response rates (P = 0.00002) and had a possible moderate effect on overall survival time (P = 0.024). Hemorrhage, thromboembolic complications, and gastrointestinal toxicities were the most frequently reported side effects.
Conclusion: The combination of bevacizumab and irinotecan might improve outcome in patients with recurrent malignant glioma. Randomized controlled trials are recommended to evaluate this treatment protocol and the additional value of irinotecan.
Figures

References
-
- Stupp R, Mason WP, den Bent MJ v, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–93. doi: 10.1054/bjoc.2000.1316. - DOI - PMC - PubMed
-
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

