A generalized physiologically-based toxicokinetic modeling system for chemical mixtures containing metals
- PMID: 20525215
- PMCID: PMC2903511
- DOI: 10.1186/1742-4682-7-17
A generalized physiologically-based toxicokinetic modeling system for chemical mixtures containing metals
Abstract
Background: Humans are routinely and concurrently exposed to multiple toxic chemicals, including various metals and organics, often at levels that can cause adverse and potentially synergistic effects. However, toxicokinetic modeling studies of exposures to these chemicals are typically performed on a single chemical basis. Furthermore, the attributes of available models for individual chemicals are commonly estimated specifically for the compound studied. As a result, the available models usually have parameters and even structures that are not consistent or compatible across the range of chemicals of concern. This fact precludes the systematic consideration of synergistic effects, and may also lead to inconsistencies in calculations of co-occurring exposures and corresponding risks. There is a need, therefore, for a consistent modeling framework that would allow the systematic study of cumulative risks from complex mixtures of contaminants.
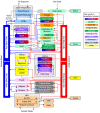
Methods: A Generalized Toxicokinetic Modeling system for Mixtures (GTMM) was developed and evaluated with case studies. The GTMM is physiologically-based and uses a consistent, chemical-independent physiological description for integrating widely varying toxicokinetic models. It is modular and can be directly "mapped" to individual toxicokinetic models, while maintaining physiological consistency across different chemicals. Interaction effects of complex mixtures can be directly incorporated into the GTMM.
Conclusions: The application of GTMM to different individual metals and metal compounds showed that it explains available observational data as well as replicates the results from models that have been optimized for individual chemicals. The GTMM also made it feasible to model toxicokinetics of complex, interacting mixtures of multiple metals and nonmetals in humans, based on available literature information. The GTMM provides a central component in the development of a "source-to-dose-to-effect" framework for modeling population health risks from environmental contaminants. As new data become available on interactions of multiple chemicals, the GTMM can be iteratively parameterized to improve mechanistic understanding of human health risks from exposures to complex mixtures of chemicals.
Figures




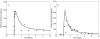
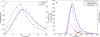

Similar articles
-
Advancing understanding of human variability through toxicokinetic modeling, in vitro-in vivo extrapolation, and new approach methodologies.Hum Genomics. 2024 Nov 21;18(1):129. doi: 10.1186/s40246-024-00691-9. Hum Genomics. 2024. PMID: 39574200 Free PMC article. Review.
-
Physiological modeling of toxicokinetic interactions: implications for mixture risk assessment.Environ Health Perspect. 1998 Dec;106 Suppl 6(Suppl 6):1377-84. doi: 10.1289/ehp.98106s61377. Environ Health Perspect. 1998. PMID: 9860896 Free PMC article. Review.
-
Development and intercomparison of single and multicompartment physiologically-based toxicokinetic models: Implications for model selection and tiered modeling frameworks.Environ Int. 2021 Sep;154:106557. doi: 10.1016/j.envint.2021.106557. Epub 2021 Apr 23. Environ Int. 2021. PMID: 33892222
-
Application of Biologically Based Lumping To Investigate the Toxicokinetic Interactions of a Complex Gasoline Mixture.Environ Sci Technol. 2016 Mar 15;50(6):3231-8. doi: 10.1021/acs.est.5b05648. Epub 2016 Mar 3. Environ Sci Technol. 2016. PMID: 26889718
-
Sources, pathways, and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry.J Toxicol Environ Health A. 2002 Jan 11;65(1):1-142. doi: 10.1080/152873902753338572. J Toxicol Environ Health A. 2002. PMID: 11809004 Review.
Cited by
-
Metal Concentrations in E-Cigarette Aerosol Samples: A Comparison by Device Type and Flavor.Environ Health Perspect. 2023 Dec;131(12):127004. doi: 10.1289/EHP11921. Epub 2023 Dec 4. Environ Health Perspect. 2023. PMID: 38048100 Free PMC article.
-
A Predictive Toxicokinetic Model for Nickel Leaching from Vascular Stents.ACS Biomater Sci Eng. 2024 Apr 8;10(4):2534-2551. doi: 10.1021/acsbiomaterials.3c01436. Epub 2024 Mar 25. ACS Biomater Sci Eng. 2024. PMID: 38525821 Free PMC article.
-
Exposure indices for the National Children's Study: application to inhalation exposures in Queens County, NY.J Expo Sci Environ Epidemiol. 2013 Jan-Feb;23(1):22-31. doi: 10.1038/jes.2012.99. Epub 2012 Oct 17. J Expo Sci Environ Epidemiol. 2013. PMID: 23072768 Free PMC article.
-
A Physiologically Based Pharmacokinetic (PBPK) Modeling Framework for Mixtures of Dioxin-like Compounds.Toxics. 2022 Nov 17;10(11):700. doi: 10.3390/toxics10110700. Toxics. 2022. PMID: 36422908 Free PMC article.
-
Risk assessment of occupational exposure to heavy metal mixtures: a study protocol.BMC Public Health. 2018 Mar 5;18(1):314. doi: 10.1186/s12889-018-5191-5. BMC Public Health. 2018. PMID: 29506513 Free PMC article.
References
-
- US Environmental Protection Agency (EPA) Approaches for the Application of Physiologically Based Pharmacokinetic (PBPK) Models and Supporting Data in Risk Assessment (Final Report) National Center for Environmental Assessment, Washington, D.C; 2006. Tech. Rep. EPA/600/R-05/043F.
-
- Isukapalli S, Roy A, Georgopoulos P. In: Pharmacometrics: the Science of Quantitative Pharmacology. Ette E, Williams PJ, Wiley, editor. 2007. Physiologically Based Pharmacokinetic Modeling: Inhalation, Ingestion and Dermal Absorption.
-
- Georgopoulos PG. A Multiscale Approach for Assessing the Interactions of Environmental and Biological Systems in a Holistic Health Risk Assessment Framework. Water, Air, & Soil Pollution: Focus. 2008;8:3–21.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

