Global utilization of low-dose corticosteroids in severe sepsis and septic shock: a report from the PROGRESS registry
- PMID: 20525247
- PMCID: PMC2911744
- DOI: 10.1186/cc9044
Global utilization of low-dose corticosteroids in severe sepsis and septic shock: a report from the PROGRESS registry
Abstract
Introduction: The benefits and use of low-dose corticosteroids (LDCs) in severe sepsis and septic shock remain controversial. Surviving sepsis campaign guidelines suggest LDC use for septic shock patients poorly responsive to fluid resuscitation and vasopressor therapy. Their use is suspected to be wide-spread, but paucity of data regarding global practice exists. The purpose of this study was to compare baseline characteristics and clinical outcomes of patients treated or not treated with LDC from the international PROGRESS (PROmoting Global Research Excellence in Severe Sepsis) cohort study of severe sepsis.
Methods: Patients enrolled in the PROGRESS registry were evaluated for use of vasopressor and LDC (equivalent or lesser potency to hydrocortisone 50 mg six-hourly plus 50 microg 9-alpha-fludrocortisone) for treatment of severe sepsis at any time in intensive care units (ICUs). Baseline characteristics and hospital mortality were analyzed, and logistic regression techniques used to develop propensity score and outcome models adjusted for baseline imbalances between groups.
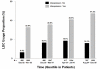
Results: A total of 8,968 patients with severe sepsis and sufficient data for analysis were studied. A total of 79.8% (7,160/8,968) of patients received vasopressors, and 34.0% (3,051/8,968) of patients received LDC. Regional use of LDC was highest in Europe (51.1%) and lowest in Asia (21.6%). Country use was highest in Brazil (62.9%) and lowest in Malaysia (9.0%). A total of 14.2% of patients on LDC were not receiving any vasopressor therapy. LDC patients were older, had more co-morbidities and higher disease severity scores. Patients receiving LDC spent longer in ICU than patients who did not (median of 12 versus 8 days; P <0.001). Overall hospital mortality rates were greater in the LDC than in the non-LDC group (58.0% versus 43.0%; P <0.001). After adjusting for baseline imbalances, in all mortality models (with vasopressor use), a consistent association remained between LDC and hospital mortality (odds ratios varying from 1.30 to 1.47).
Conclusions: Widespread use of LDC for the treatment of severe sepsis with significant regional and country variation exists. In this study, 14.2% of patients received LDC despite the absence of evidence of shock. Hospital mortality was higher in the LDC group and remained higher after adjustment for key determinates of mortality.
Figures
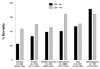
Comment in
-
Corticosteroids for sepsis: registry versus Cochrane systematic review!Crit Care. 2010;14(4):185. doi: 10.1186/cc9188. Epub 2010 Jul 30. Crit Care. 2010. PMID: 20727225 Free PMC article.
References
-
- Schein RM, Sprung CL. Critical Care-State of the Art 1986. Vol. 7. The Society of Critical Care Medicine: Fullerton; 1986. The use of corticosteroids in the sepsis syndrome. pp. 131–149.
-
- Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

