Antisense oligonucleotide targeting Livin induces apoptosis of human bladder cancer cell via a mechanism involving caspase 3
- PMID: 20525250
- PMCID: PMC2890551
- DOI: 10.1186/1756-9966-29-63
Antisense oligonucleotide targeting Livin induces apoptosis of human bladder cancer cell via a mechanism involving caspase 3
Abstract
Background and aim: in recent years, Livin, a new member of IAPs family, is found to be a key molecule in cancers. Researchers consider Livin may become a new target for tumor therapy; however, the role of it in bladder cancer is still unclear. The purpose of this article is to investigate Antisense Oligonucleotide (ASODN) of Livin on treating bladder cancer cell and underlying mechanisms.
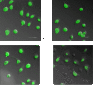
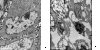
Methods: Phosphorathioate modifying was used to synthesize antisense oligonucleotides targeting Livin, followed by transfection into human bladder cancer cell 5637. After transfection, Livin mRNA and protein level, cell proliferation and apoptosis changes, caspase3 level and its effect on human bladder cancer transplantable tumor in nude mice were measured.
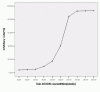
Result: results showed Livin ASODN effectively inhibited Livin expression and tumor cell proliferation, and these effects probably through enhanced caspase3 activity and apoptosis of tumor cells. In nude mice transplantable tumor model, Livin expressions were inhibited meanwhile caspase3 expression was increased. Tumor growth slowed down and apoptosis was enhanced.
Conclusion: Our data suggest that Livin plays an important role in inhibiting apoptosis of bladder cancer cells. Livin ASODN may promote cell apoptosis, inhibit bladder cancer growth, and become one of the methods of gene therapy for bladder cancer.
Figures






Similar articles
-
Livin may serve as a marker for prognosis of bladder cancer relapse and a target of bladder cancer treatment.Urol Oncol. 2009 May-Jun;27(3):277-83. doi: 10.1016/j.urolonc.2008.03.015. Epub 2008 Jun 16. Urol Oncol. 2009. PMID: 18555709
-
Silencing Livin induces apoptotic and autophagic cell death, increasing chemotherapeutic sensitivity to cisplatin of renal carcinoma cells.Tumour Biol. 2016 Nov;37(11):15133-15143. doi: 10.1007/s13277-016-5395-1. Epub 2016 Sep 27. Tumour Biol. 2016. PMID: 27677286
-
siRNA directed against Livin inhibits tumor growth and induces apoptosis in human glioma cells.J Neurooncol. 2012 Mar;107(1):81-7. doi: 10.1007/s11060-011-0728-9. Epub 2011 Nov 16. J Neurooncol. 2012. PMID: 22086237
-
Research progress on Livin protein: an inhibitor of apoptosis.Mol Cell Biochem. 2011 Nov;357(1-2):39-45. doi: 10.1007/s11010-011-0873-7. Epub 2011 May 27. Mol Cell Biochem. 2011. PMID: 21617971 Review.
-
Network Pharmacology Research and Dual-omic Analyses Reveal the Molecular Mechanism of Natural Product Nodosin Inhibiting Muscle-Invasive Bladder Cancer in Vitro and in Vivo.J Nat Prod. 2022 Aug 26;85(8):2006-2017. doi: 10.1021/acs.jnatprod.2c00400. Epub 2022 Aug 17. J Nat Prod. 2022. PMID: 35976233 Review.
Cited by
-
RNA-seq reveals novel mechanistic targets of Livin in bladder cancer.BMC Urol. 2023 Feb 28;23(1):26. doi: 10.1186/s12894-023-01194-w. BMC Urol. 2023. PMID: 36855119 Free PMC article.
-
Livin/BIRC7 expression as malignancy marker in adrenocortical tumors.Oncotarget. 2017 Feb 7;8(6):9323-9338. doi: 10.18632/oncotarget.14067. Oncotarget. 2017. PMID: 28030838 Free PMC article.
-
An investigation into the cytotoxic effects of 13-acetoxysarcocrassolide from the soft coral Sarcophyton crassocaule on bladder cancer cells.Mar Drugs. 2011 Dec;9(12):2622-2642. doi: 10.3390/md9122622. Epub 2011 Dec 13. Mar Drugs. 2011. PMID: 22363243 Free PMC article.
-
Livin, Survivin and Caspase 3 as early recurrence markers in non-muscle-invasive bladder cancer.World J Urol. 2014 Dec;32(6):1477-84. doi: 10.1007/s00345-014-1246-0. Epub 2014 Mar 5. World J Urol. 2014. PMID: 24595485
-
Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway.Int J Mol Sci. 2012;13(2):2025-2035. doi: 10.3390/ijms13022025. Epub 2012 Feb 14. Int J Mol Sci. 2012. PMID: 22408435 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

