A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season
- PMID: 20525274
- PMCID: PMC2898783
- DOI: 10.1186/1471-2431-10-38
A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season
Abstract
Background: Respiratory syncytial virus (RSV) is an important pathogen causing annual epidemics of bronchiolitis and pneumonia among infants worldwide. High-risk infants currently receive RSV prophylaxis with palivizumab, a humanized RSV monoclonal antibody (MAb). In preclinical in vitro and in vivo (cotton-rat model) studies, motavizumab, a new RSV MAb, was shown to have greater anti-RSV activity than palivizumab. Motavizumab is currently under review for licensing approval. Since both MAbs may be available concurrently, this study evaluated their safety and tolerability when administered sequentially during the same RSV season.
Methods: Between April 2006 and May 2006, 260 high-risk infants were randomly assigned 1:1:1 to receive monthly intramuscular injections: 2 doses of motavizumab followed by 3 doses of palivizumab (M/P); 2 doses of palivizumab followed by 3 doses of motavizumab (P/M); or 5 doses of motavizumab (control). Adverse events (AEs, serious AEs [SAEs]), development of antidrug antibody (ADA), and serum drug trough concentrations were assessed.
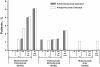
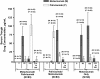
Results: Most children received all 5 doses (246/260 [94.6%]) and completed the study (241/260 [92.7%]). While overall AE rates were similar (mostly level 1 or 2 in severity), SAEs and level 3 AEs occurred more frequently in the M/P group (SAEs: 22.9% M/P, 8.4% P/M, 11.8% motavizumab only; level 3 AEs: 15.7% M/P, 6.0% P/M, 6.5% motavizumab only). This trend in AE rates occurred before and after switching from motavizumab to palivizumab, suggesting a cause other than the combined regimen. Frequencies of AEs judged by the investigator to be related to study drug were similar among groups. Two deaths occurred on study (both in the M/P group, before palivizumab administration); neither was considered by the site investigator to be related to study drug. Mean serum drug trough concentrations were comparable among groups; ADA detection was infrequent (5.1% or less of any group).
Conclusions: The conclusions drawn from this study are limited by the small sample size per group. However, within this small study, overall AE rates, serum drug trough concentrations, and development of ADA associated with administering motavizumab and palivizumab sequentially to high-risk children appear comparable to administering motavizumab alone during the same RSV season.
Trial registration: clinicaltrials.gov NCT00316264.
Figures

References
-
- Hall CB, Hall WJ. In: Principles and Practice of Infectious Diseases. 4. Mandell GL, Bennett JE, Dolin R, editor. New York: Churchill Livingstone; 1995. Bronchiolitis; pp. 612–619.
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child (1960) 1986;140:543–546. - PubMed
-
- Groothuis JR, Gutierrez KM, Lauer BA. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics. 1988;82:199–203. - PubMed
-
- Heilman CA. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–406. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

