Immunization with a dominant-negative recombinant Herpes Simplex Virus (HSV) type 1 protects against HSV-2 genital disease in guinea pigs
- PMID: 20525279
- PMCID: PMC2889954
- DOI: 10.1186/1471-2180-10-163
Immunization with a dominant-negative recombinant Herpes Simplex Virus (HSV) type 1 protects against HSV-2 genital disease in guinea pigs
Abstract
Background: CJ9-gD is a novel dominant-negative recombinant herpes simplex virus type 1 (HSV-1) that is completely replication-defective, cannot establish detectable latent infection in vivo, and expresses high levels of the major HSV-1 antigen glycoprotein D immediately following infection. In the present study, CJ9-gD was evaluated as a vaccine against HSV-2 genital infection in guinea pigs.
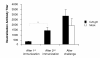
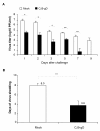
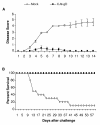
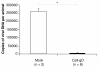
Results: Animals immunized with CJ9-gD developed at least 700-fold higher titers of HSV-2-specific neutralization antibodies than mock-immunized controls. After challenge with wild-type HSV-2, all 10 control guinea pigs developed multiple genital lesions with an average of 21 lesions per animal. In contrast, only 2 minor lesions were found in 2 of 8 CJ9-gD-immunized animals, representing a 40-fold reduction on the incidence of primary genital lesions in immunized animals (p < 0.0001). Immunization significantly reduced the amount and duration of viral shedding and provided complete protection against neurological symptoms, while 90% of mock-immunized animals succumbed due to the severity of disease. Importantly, immunized animals showed no signs of recurrent disease or viral shedding during a 60-days observation period after recovery from primary infection, and carried 50-fold less latent viral DNA load in their dorsal root ganglia than the surviving mock-vaccinated controls (p < 0.0001).
Conclusions: Collectively, we demonstrate that vaccination with the HSV-1 recombinant CJ9-gD elicits strong and protective immune responses against primary and recurrent HSV-2 genital disease and significantly reduces the extent of latent infection.
Figures

Similar articles
-
Immunization with a dominant-negative recombinant HSV type 1 protects against HSV-1 skin disease in guinea pigs.J Invest Dermatol. 2008 Dec;128(12):2825-32. doi: 10.1038/jid.2008.142. Epub 2008 May 22. J Invest Dermatol. 2008. PMID: 18496565
-
Prevention of genital herpes simplex virus type 1 and 2 disease in mice immunized with a gD-expressing dominant-negative recombinant HSV-1.J Invest Dermatol. 2009 Oct;129(10):2470-9. doi: 10.1038/jid.2009.86. Epub 2009 Apr 9. J Invest Dermatol. 2009. PMID: 19357711 Free PMC article.
-
Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone.J Virol. 2011 Oct;85(20):10472-86. doi: 10.1128/JVI.00849-11. Epub 2011 Aug 3. J Virol. 2011. PMID: 21813597 Free PMC article.
-
The challenge of developing a herpes simplex virus 2 vaccine.Expert Rev Vaccines. 2012 Dec;11(12):1429-40. doi: 10.1586/erv.12.129. Expert Rev Vaccines. 2012. PMID: 23252387 Free PMC article. Review.
-
Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?Vaccine. 2011 Aug 11;29(35):5824-36. doi: 10.1016/j.vaccine.2011.06.083. Epub 2011 Jun 28. Vaccine. 2011. PMID: 21718746 Free PMC article. Review.
Cited by
-
Defective natural killer cell activity in a mouse model of eczema herpeticum.J Allergy Clin Immunol. 2017 Mar;139(3):997-1006.e10. doi: 10.1016/j.jaci.2016.06.034. Epub 2016 Jul 29. J Allergy Clin Immunol. 2017. PMID: 27476888 Free PMC article.
-
A herpes simplex virus 2 (HSV-2) glycoprotein D-expressing nonreplicating dominant-negative HSV-2 virus vaccine is superior to a gD2 subunit vaccine against HSV-2 genital infection in guinea pigs.PLoS One. 2014 Jun 30;9(6):e101373. doi: 10.1371/journal.pone.0101373. eCollection 2014. PLoS One. 2014. PMID: 24979708 Free PMC article.
-
Herpes Simplex Vaccines: Prospects of Live-attenuated HSV Vaccines to Combat Genital and Ocular infections.Curr Clin Microbiol Rep. 2015 Sep 1;2(3):125-136. doi: 10.1007/s40588-015-0020-4. Epub 2015 Jul 1. Curr Clin Microbiol Rep. 2015. PMID: 27114893 Free PMC article.
-
Pan-HSV-2 IgG antibody in vaccinated mice and guinea pigs correlates with protection against herpes simplex virus 2.PLoS One. 2013 Jun 6;8(6):e65523. doi: 10.1371/journal.pone.0065523. Print 2013. PLoS One. 2013. PMID: 23755244 Free PMC article.
-
Prophylactic and therapeutic modulation of innate and adaptive immunity against mucosal infection of herpes simplex virus.Immune Netw. 2014 Aug;14(4):187-200. doi: 10.4110/in.2014.14.4.187. Epub 2014 Aug 22. Immune Netw. 2014. PMID: 25177251 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

