Biotinylated-sortase self-cleavage purification (BISOP) method for cell-free produced proteins
- PMID: 20525307
- PMCID: PMC2901362
- DOI: 10.1186/1472-6750-10-42
Biotinylated-sortase self-cleavage purification (BISOP) method for cell-free produced proteins
Abstract
Background: Technology used for the purification of recombinant proteins is a key issue for the biochemical and structural analyses of proteins. In general, affinity tags, such as glutathione-S-transferase or six-histidines, are used to purify recombinant proteins. Since such affinity tags often interfere negatively with the structural and functional analyses of proteins, they are usually removed by treatment with proteases. Previously, Dr. H. Mao reported self-cleavage purification of a target protein by fusing the sortase protein to its N-terminal end, and subsequently obtained tag-free recombinant protein following expression in Escherichia coli. This method, however, is yet to be applied to the cell-free based protein production.
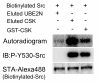
Results: The histidine tag-based self-cleavage method for purifying proteins produced by the wheat cell-free protein synthesis system showed high background, low recovery, and unexpected cleavage between the N-terminally fused sortase and target protein during the protein synthesis. Addition of calcium chelator BAPTA to the cell-free reaction inhibited the cleavage. In order to adapt the sortase-based purification method to the cell-free system, we next used biotin as the affinity tag. The biotinylated sortase self-cleavage purification (BISOP) method provided tag-free, highly purified proteins due to improved recovery of proteins from the resin. The N-terminal sequence analysis of the GFP produced by the BISOP method revealed that the cleavage indeed occurred at the right cleavage site. Using this method, we also successfully purified the E2 heterocomplex of USE2N and USE2v1. The c-terminal src kinase (CSK) obtained by the BISOP method showed high activity in phosphorylating the Src protein. Furthermore, we demonstrated that this method is suitable for automatically synthesizing and purifying proteins using robots.
Conclusion: We demonstrated that the newly developed BISOP method is very useful for obtaining high quality, tag-free recombinant proteins, produced using the cell-free system, for biochemical and structural analyses.
Figures



Similar articles
-
A new vector coupling ligation-independent cloning with sortase a fusion for efficient cloning and one-step purification of tag-free recombinant proteins.Protein Expr Purif. 2019 Sep;161:1-7. doi: 10.1016/j.pep.2019.04.004. Epub 2019 Apr 22. Protein Expr Purif. 2019. PMID: 31022449
-
Purification of E. coli proteins using a self-cleaving chitin-binding affinity tag.Methods Mol Biol. 2014;1177:47-58. doi: 10.1007/978-1-4939-1034-2_4. Methods Mol Biol. 2014. PMID: 24943313
-
Influence of the protein oligomericity on final yield after affinity tag removal in purification of recombinant proteins.J Chromatogr A. 2006 Jan 6;1101(1-2):293-306. doi: 10.1016/j.chroma.2005.09.089. Epub 2005 Oct 26. J Chromatogr A. 2006. PMID: 16256128
-
Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins.Protein Expr Purif. 2006 Jul;48(1):1-13. doi: 10.1016/j.pep.2005.12.002. Epub 2005 Dec 28. Protein Expr Purif. 2006. PMID: 16427311 Review.
-
Aggregating tags for column-free protein purification.Biotechnol J. 2015 Dec;10(12):1877-86. doi: 10.1002/biot.201500299. Epub 2015 Nov 11. Biotechnol J. 2015. PMID: 26556016 Review.
Cited by
-
Production of monoclonal antibodies against GPCR using cell-free synthesized GPCR antigen and biotinylated liposome-based interaction assay.Sci Rep. 2015 Jun 10;5:11333. doi: 10.1038/srep11333. Sci Rep. 2015. PMID: 26061673 Free PMC article.
-
In vivo polyester immobilized sortase for tagless protein purification.Microb Cell Fact. 2015 Nov 25;14:190. doi: 10.1186/s12934-015-0385-3. Microb Cell Fact. 2015. PMID: 26608345 Free PMC article.
-
Bacterial inclusion bodies as potential synthetic devices for pathogen recognition and a therapeutic substance release.Microb Cell Fact. 2013 Feb 7;12:16. doi: 10.1186/1475-2859-12-16. Microb Cell Fact. 2013. PMID: 23391325 Free PMC article.
-
Recent advances in sortase-catalyzed ligation methodology.Curr Opin Struct Biol. 2016 Jun;38:111-8. doi: 10.1016/j.sbi.2016.05.021. Epub 2016 Jun 16. Curr Opin Struct Biol. 2016. PMID: 27318815 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

