Anti-angiogenic SPARC peptides inhibit progression of neuroblastoma tumors
- PMID: 20525313
- PMCID: PMC2895596
- DOI: 10.1186/1476-4598-9-138
Anti-angiogenic SPARC peptides inhibit progression of neuroblastoma tumors
Abstract
Background: New, more effective strategies are needed to treat highly aggressive neuroblastoma. Our laboratory has previously shown that full-length Secreted Protein Acidic and Rich in Cysteine (SPARC) and a SPARC peptide corresponding to the follistatin domain of the protein (FS-E) potently block angiogenesis and inhibit the growth of neuroblastoma tumors in preclinical models. Peptide FS-E is structurally complex and difficult to produce, limiting its potential as a therapeutic in the clinic.
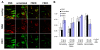
Results: In this study, we synthesized two smaller and structurally more simple SPARC peptides, FSEN and FSEC, that respectively correspond to the N-and C-terminal loops of peptide FS-E. We show that both peptides FSEN and FSEC have anti-angiogenic activity in vitro and in vivo, although FSEC is more potent. Peptide FSEC also significantly inhibited the growth of neuroblastoma xenografts. Histologic examination demonstrated characteristic features of tumor angiogenesis with structurally abnormal, tortuous blood vessels in control neuroblastoma xenografts. In contrast, the blood vessels observed in tumors, treated with SPARC peptides, were thin walled and structurally more normal. Using a novel method to quantitatively assess blood vessel abnormality we demonstrated that both SPARC peptides induced changes in blood vessel architecture that are consistent with blood vessel normalization.
Conclusion: Our results demonstrate that SPARC peptide FSEC has potent anti-angiogenic and anti-tumorigenic effects in neuroblastoma. Its simple structure and ease of production indicate that it may have clinical utility in the treatment of high-risk neuroblastoma and other types of pediatric and adult cancers, which depend on angiogenesis.
Figures

Similar articles
-
Notch signaling regulates tumor-induced angiogenesis in SPARC-overexpressed neuroblastoma.Angiogenesis. 2013 Jan;16(1):85-100. doi: 10.1007/s10456-012-9301-1. Epub 2012 Sep 6. Angiogenesis. 2013. PMID: 22956186 Free PMC article.
-
Neuroblastoma angiogenesis is inhibited with a folded synthetic molecule corresponding to the epidermal growth factor-like module of the follistatin domain of SPARC.Cancer Res. 2004 Oct 15;64(20):7420-5. doi: 10.1158/0008-5472.CAN-04-2141. Cancer Res. 2004. PMID: 15492265
-
SPARC is a key Schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis.Cancer Res. 2002 Dec 15;62(24):7357-63. Cancer Res. 2002. PMID: 12499280
-
Recent advances in targeted anti-vasculature therapy: the neuroblastoma model.Curr Drug Targets. 2009 Oct;10(10):1021-7. doi: 10.2174/138945009789577954. Curr Drug Targets. 2009. PMID: 19663770 Review.
-
Angiogenesis in neuroblastoma.Ann N Y Acad Sci. 2004 Dec;1028:133-42. doi: 10.1196/annals.1322.014. Ann N Y Acad Sci. 2004. PMID: 15650239 Review.
Cited by
-
Notch signaling regulates tumor-induced angiogenesis in SPARC-overexpressed neuroblastoma.Angiogenesis. 2013 Jan;16(1):85-100. doi: 10.1007/s10456-012-9301-1. Epub 2012 Sep 6. Angiogenesis. 2013. PMID: 22956186 Free PMC article.
-
The SPARC protein: an overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease.Br J Pharmacol. 2017 Jan;174(1):3-14. doi: 10.1111/bph.13653. Epub 2016 Nov 25. Br J Pharmacol. 2017. PMID: 27759879 Free PMC article. Review.
-
Cooperative Cross-Talk between Neuroblastoma Subtypes Confers Resistance to Anaplastic Lymphoma Kinase Inhibition.Genes Cancer. 2011 May;2(5):538-49. doi: 10.1177/1947601911416003. Genes Cancer. 2011. PMID: 21901167 Free PMC article.
-
Deficiency of Stabilin-1 in the Context of Hepatic Melanoma Metastasis.Cancers (Basel). 2024 Jan 19;16(2):441. doi: 10.3390/cancers16020441. Cancers (Basel). 2024. PMID: 38275881 Free PMC article.
-
Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK.Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):15999-6004. doi: 10.1073/pnas.1419057111. Epub 2014 Oct 31. Proc Natl Acad Sci U S A. 2014. PMID: 25362046 Free PMC article.
References
-
- Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc amplification, and poor outcome in human neuroblastoma. J Clin Oncol. 1996;14:405–414. - PubMed
-
- Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–8. - PubMed
-
- Katzenstein HM, Rademaker AW, Senger C, Salwen HR, Nguyen NN, Thorner PS, Litsas L, Cohn SL. Effectiveness of the angiogenesis inhibitor TNP-470 in reducing the growth of human neuroblastoma in nude mice inversely correlates with tumor burden. Clin Cancer Res. 1999;5:4273–4278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

