In vitro and in vivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillin
- PMID: 20525378
- PMCID: PMC2897798
- DOI: 10.1186/1471-2334-10-153
In vitro and in vivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillin
Abstract
Background: Oxacillin continues to be an important agent in the treatment of staphylococcal infections; many generic products are available and the only requirement for their approval is demonstration of pharmaceutical equivalence. We tested the assumption that pharmaceutical equivalence predicts therapeutic equivalence by comparing 11 generics with the innovator product in terms of concentration of the active pharmaceutical ingredient (API), minimal inhibitory (MIC) and bactericidal concentrations (MBC), and antibacterial efficacy in the neutropenic mouse thigh infection model.
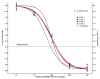
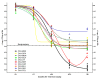
Methods: The API in each product was measured by a validated microbiological assay and compared by slope (potency) and intercept (concentration) analysis of linear regressions. MIC and MBC were determined by broth microdilution according to Clinical and Laboratory Standard Institute (CLSI) guidelines. For in vivo efficacy, neutropenic ICR mice were inoculated with a clinical strain of Staphylococcus aureus. The animals had 4.14 +/- 0.18 log10 CFU/thigh when treatment started. Groups of 10 mice per product received a total dose ranging from 2.93 to 750 mg/kg per day administered q1h. Sigmoidal dose-response curves were generated by nonlinear regression fitted to Hill equation to compute maximum effect (Emax), slope (N), and the effective dose reaching 50% of the Emax (ED50). Based on these results, bacteriostatic dose (BD) and dose needed to kill the first log of bacteria (1LKD) were also determined.
Results: 4 generic products failed pharmaceutical equivalence due to significant differences in potency; however, all products were undistinguishable from the innovator in terms of MIC and MBC. Independently of their status with respect to pharmaceutical equivalence or in vitro activity, all generics failed therapeutic equivalence in vivo, displaying significantly lower Emax and requiring greater BD and 1LKD, or fitting to a non-sigmoidal model.
Conclusions: Pharmaceutical or in vitro equivalence did not entail therapeutic equivalence for oxacillin generic products, indicating that criteria for approval deserve review to include evaluation of in vivo efficacy.
Figures
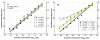
Similar articles
-
Therapeutic equivalence requires pharmaceutical, pharmacokinetic, and pharmacodynamic identities: true bioequivalence of a generic product of intravenous metronidazole.Antimicrob Agents Chemother. 2012 May;56(5):2659-65. doi: 10.1128/AAC.06012-11. Epub 2012 Feb 13. Antimicrob Agents Chemother. 2012. PMID: 22330928 Free PMC article.
-
Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator.Antimicrob Agents Chemother. 2010 Aug;54(8):3271-9. doi: 10.1128/AAC.01044-09. Epub 2010 Jun 14. Antimicrob Agents Chemother. 2010. PMID: 20547818 Free PMC article.
-
Nontherapeutic equivalence of a generic product of imipenem-cilastatin is caused more by chemical instability of the active pharmaceutical ingredient (imipenem) than by its substandard amount of cilastatin.PLoS One. 2019 Feb 6;14(2):e0211096. doi: 10.1371/journal.pone.0211096. eCollection 2019. PLoS One. 2019. PMID: 30726248 Free PMC article.
-
Acceptability of generic versus innovator oral medicines: not only a matter of taste.Drug Discov Today. 2021 Feb;26(2):329-343. doi: 10.1016/j.drudis.2020.11.008. Epub 2020 Nov 17. Drug Discov Today. 2021. PMID: 33217597 Review.
-
Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation.Eur J Hosp Pharm. 2013 Oct;20(5):302-307. doi: 10.1136/ejhpharm-2012-000258. Epub 2013 Aug 29. Eur J Hosp Pharm. 2013. PMID: 24089632 Free PMC article. Review.
Cited by
-
Therapeutic equivalence requires pharmaceutical, pharmacokinetic, and pharmacodynamic identities: true bioequivalence of a generic product of intravenous metronidazole.Antimicrob Agents Chemother. 2012 May;56(5):2659-65. doi: 10.1128/AAC.06012-11. Epub 2012 Feb 13. Antimicrob Agents Chemother. 2012. PMID: 22330928 Free PMC article.
-
Serum albumin and α-1 acid glycoprotein impede the killing of Schistosoma mansoni by the tyrosine kinase inhibitor Imatinib.Int J Parasitol Drugs Drug Resist. 2014 Aug 7;4(3):287-95. doi: 10.1016/j.ijpddr.2014.07.005. eCollection 2014 Dec. Int J Parasitol Drugs Drug Resist. 2014. PMID: 25516839 Free PMC article.
-
Pharmacodynamics of nine generic products of amikacin compared with the innovator in the neutropenic mouse thigh infection model.BMC Res Notes. 2015 Oct 7;8:546. doi: 10.1186/s13104-015-1507-z. BMC Res Notes. 2015. PMID: 26445936 Free PMC article.
-
Myths and Misconceptions around Antibiotic Resistance: Time to Get Rid of Them.Infect Chemother. 2022 Sep;54(3):393-408. doi: 10.3947/ic.2022.0060. Epub 2022 Aug 5. Infect Chemother. 2022. PMID: 36047302 Free PMC article. Review.
-
Cefazolin high-inoculum effect in methicillin-susceptible Staphylococcus aureus from South American hospitals.J Antimicrob Chemother. 2013 Dec;68(12):2773-8. doi: 10.1093/jac/dkt254. Epub 2013 Jun 21. J Antimicrob Chemother. 2013. PMID: 23794599 Free PMC article.
References
-
- Food and Drug Administration (FDA) Guidance for industry. Statistical Approach to Establishing Bioequivalence. 2009.
-
- United States Pharmacopeial Convention (USP) United States Pharmacopeia and National Formulary. Rockville, MD: USP; 1999.
-
- Rodriguez CA, Zuluaga AF, Salazar BE, Agudelo M, Vesga O. Experimental comparison of 11 generic products of oxacillin with the original compound in terms of concentration of active principle, in vitro activity, and in vivo efficacy, using the neutropenic murine thigh infection model [Abstract A-1305] Abstracts of the 44th ICAAC. 2004. pp. 28–29.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

