Barbiturates require the N terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta GABAA receptor currents
- PMID: 20525684
- PMCID: PMC2911329
- DOI: 10.1074/jbc.M110.122564
Barbiturates require the N terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta GABAA receptor currents
Abstract
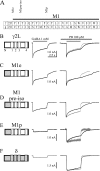
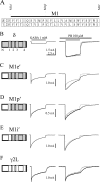
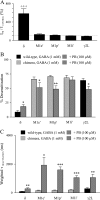
GABA(A) receptors are composed predominantly of alphabetagamma receptors, which mediate primarily synaptic inhibition, and alphabetadelta receptors, which mediate primarily extrasynaptic inhibition. At saturating GABA concentrations, the barbiturate pentobarbital substantially increased the amplitude and desensitization of the alpha1beta3delta receptor but not the alpha1beta3gamma2L receptor currents. To explore the structural domains of the delta subunit that are involved in pentobarbital potentiation and increased desensitization of alpha1beta3delta currents, chimeric cDNAs were constructed by progressive replacement of gamma2L subunit sequence with a delta subunit sequence or a delta subunit sequence with a gamma2L subunit sequence, and HEK293T cells were co-transfected with alpha1 and beta3 subunits or alpha1 and beta3 subunits and a gamma2L, delta, or chimeric subunit. Currents evoked by a saturating concentration of GABA or by co-application of GABA and pentobarbital were recorded using the patch clamp technique. By comparing the extent of enhancement and changes in kinetic properties produced by pentobarbital among chimeric and wild type receptors, we concluded that although potentiation of alpha1beta3delta currents by pentobarbital required the delta subunit sequence from the N terminus to proline 241 in the first transmembrane domain (M1), increasing desensitization of alpha1beta3delta currents required a delta subunit sequence from the N terminus to isoleucine 235 in M1. These findings suggest that the delta subunit N terminus and N-terminal portion of the M1 domain are, at least in part, involved in transduction of the allosteric effect of pentobarbital to enhance alpha1beta3delta currents and that this effect involves a distinct but overlapping structural domain from that involved in altering desensitization.
Figures

Similar articles
-
Etomidate produces similar allosteric modulation in α1β3δ and α1β3γ2L GABA(A) receptors.Br J Pharmacol. 2014 Feb;171(3):789-98. doi: 10.1111/bph.12507. Br J Pharmacol. 2014. PMID: 24199598 Free PMC article.
-
Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function.J Physiol. 2002 Oct 1;544(Pt 1):3-18. doi: 10.1113/jphysiol.2002.020255. J Physiol. 2002. PMID: 12356876 Free PMC article.
-
Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2L GABAA receptor currents.Mol Pharmacol. 2004 Oct;66(4):988-1003. doi: 10.1124/mol.104.002543. Epub 2004 Jul 9. Mol Pharmacol. 2004. PMID: 15247320
-
Comparison of αβδ and αβγ GABAA receptors: Allosteric modulation and identification of subunit arrangement by site-selective general anesthetics.Pharmacol Res. 2018 Jul;133:289-300. doi: 10.1016/j.phrs.2017.12.031. Epub 2017 Dec 30. Pharmacol Res. 2018. PMID: 29294355 Free PMC article. Review.
-
Molecular and Regulatory Mechanisms of Desensitization and Resensitization of GABAA Receptors with a Special Reference to Propofol/Barbiturate.Int J Mol Sci. 2020 Jan 15;21(2):563. doi: 10.3390/ijms21020563. Int J Mol Sci. 2020. PMID: 31952324 Free PMC article. Review.
Cited by
-
Etomidate uniquely modulates the desensitization of recombinant α1β3δ GABA(A) receptors.Neuroscience. 2015 Aug 6;300:307-13. doi: 10.1016/j.neuroscience.2015.05.051. Epub 2015 May 29. Neuroscience. 2015. PMID: 26028470 Free PMC article.
-
General Anesthetic Binding Sites in Human α4β3δ γ-Aminobutyric Acid Type A Receptors (GABAARs).J Biol Chem. 2016 Dec 16;291(51):26529-26539. doi: 10.1074/jbc.M116.753335. Epub 2016 Nov 7. J Biol Chem. 2016. PMID: 27821594 Free PMC article.
-
The Influence of AA29504 on GABAA Receptor Ligand Binding Properties and Its Implications on Subtype Selectivity.Neurochem Res. 2022 Mar;47(3):667-678. doi: 10.1007/s11064-021-03475-y. Epub 2021 Nov 2. Neurochem Res. 2022. PMID: 34727270 Free PMC article.
-
Etomidate Effects on Desensitization and Deactivation of α4β3δ GABAA Receptors Inducibly Expressed in HEK293 TetR Cells.J Pharmacol Exp Ther. 2019 Jan;368(1):100-105. doi: 10.1124/jpet.118.252403. Epub 2018 Nov 2. J Pharmacol Exp Ther. 2019. PMID: 30389723 Free PMC article.
-
Assessment of subunit-dependent direct gating and allosteric modulatory effects of carisoprodol at GABA(A) receptors.Neuropharmacology. 2015 Oct;97:414-25. doi: 10.1016/j.neuropharm.2015.04.007. Epub 2015 Apr 18. Neuropharmacology. 2015. PMID: 25896767 Free PMC article.
References
-
- Olsen R. W., Macdonald R. L. (2002) Glutamate and GABA Receptors and Transporters: Structure, Function, and Pharmacology, pp. 202–235, Taylor & Francis, London
-
- Hevers W., Lüddens H. (1998) Mol. Neurobiol. 18, 35–86 - PubMed
-
- McKernan R. M., Whiting P. J. (1996) Trends Neurosci. 19, 139–143 - PubMed
-
- Mody I., Pearce R. A. (2004) Trends Neurosci. 27, 569–575 - PubMed
-
- Farrant M., Nusser Z. (2005) Nat. Rev. Neurosci. 6, 215–229 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

