Functional and morphological adaptation to peptidoglycan precursor alteration in Lactococcus lactis
- PMID: 20525686
- PMCID: PMC2911304
- DOI: 10.1074/jbc.M110.143636
Functional and morphological adaptation to peptidoglycan precursor alteration in Lactococcus lactis
Abstract
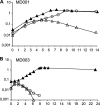
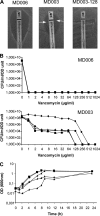
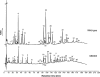
Cell wall peptidoglycan assembly is a tightly regulated process requiring the combined action of multienzyme complexes. In this study we provide direct evidence showing that substrate transformations occurring at the different stages of this process play a crucial role in the spatial and temporal coordination of the cell wall synthesis machinery. Peptidoglycan substrate alteration was investigated in the Gram-positive bacterium Lactococcus lactis by substituting the peptidoglycan precursor biosynthesis genes of this bacterium for those of the vancomycin-resistant bacterium Lactobacillus plantarum. A set of L. lactis mutant strains in which the normal d-Ala-ended precursors were partially or totally replaced by d-Lac-ended precursors was generated. Incorporation of the altered precursor into the cell wall induced morphological changes arising from a defect in cell elongation and cell separation. Structural analysis of the muropeptides confirmed that the activity of multiple enzymes involved in peptidoglycan synthesis was altered. Optimization of this altered pathway was necessary to increase the level of vancomycin resistance conferred by the utilization of d-Lac-ended peptidoglycan precursors in the mutant strains. The implications of these findings on the control of bacterial cell morphogenesis and the mechanisms of vancomycin resistance are discussed.
Figures
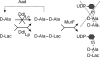


Similar articles
-
Effect of D-Ala-Ended Peptidoglycan Precursors on the Immune Regulation of Lactobacillus plantarum Strains.Front Immunol. 2022 Jan 19;12:825825. doi: 10.3389/fimmu.2021.825825. eCollection 2021. Front Immunol. 2022. PMID: 35126378 Free PMC article.
-
Another Brick in the Wall: a Rhamnan Polysaccharide Trapped inside Peptidoglycan of Lactococcus lactis.mBio. 2017 Sep 12;8(5):e01303-17. doi: 10.1128/mBio.01303-17. mBio. 2017. PMID: 28900021 Free PMC article.
-
Knockout of the two ldh genes has a major impact on peptidoglycan precursor synthesis in Lactobacillus plantarum.J Bacteriol. 1996 Sep;178(18):5431-7. doi: 10.1128/jb.178.18.5431-5437.1996. J Bacteriol. 1996. PMID: 8808932 Free PMC article.
-
Penicillin-Binding Proteins (PBPs) and Bacterial Cell Wall Elongation Complexes.Subcell Biochem. 2019;93:273-289. doi: 10.1007/978-3-030-28151-9_8. Subcell Biochem. 2019. PMID: 31939154 Review.
-
Self-resistance mechanisms of actinomycetes producing lipid II-targeting antibiotics.Int J Med Microbiol. 2015 Feb;305(2):190-5. doi: 10.1016/j.ijmm.2014.12.015. Epub 2014 Dec 23. Int J Med Microbiol. 2015. PMID: 25601631 Review.
Cited by
-
Recent advances in pneumococcal peptidoglycan biosynthesis suggest new vaccine and antimicrobial targets.Curr Opin Microbiol. 2012 Apr;15(2):194-203. doi: 10.1016/j.mib.2011.12.013. Epub 2012 Jan 24. Curr Opin Microbiol. 2012. PMID: 22280885 Free PMC article. Review.
-
Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress.Microorganisms. 2025 Mar 12;13(3):647. doi: 10.3390/microorganisms13030647. Microorganisms. 2025. PMID: 40142539 Free PMC article.
-
Deciphering the metabolism of Lactobacillus delbrueckii subsp. delbrueckii during soy juice fermentation using phenotypic and transcriptional analysis.Appl Environ Microbiol. 2024 Mar 20;90(3):e0193623. doi: 10.1128/aem.01936-23. Epub 2024 Feb 20. Appl Environ Microbiol. 2024. PMID: 38376234 Free PMC article.
-
Lysis of a Lactococcus lactis Dipeptidase Mutant and Rescue by Mutation in the Pleiotropic Regulator CodY.Appl Environ Microbiol. 2020 Apr 1;86(8):e02937-19. doi: 10.1128/AEM.02937-19. Print 2020 Apr 1. Appl Environ Microbiol. 2020. PMID: 32005740 Free PMC article.
-
Cell wall structure and function in lactic acid bacteria.Microb Cell Fact. 2014 Aug 29;13 Suppl 1(Suppl 1):S9. doi: 10.1186/1475-2859-13-S1-S9. Epub 2014 Aug 29. Microb Cell Fact. 2014. PMID: 25186919 Free PMC article. Review.
References
-
- Barreteau H., Kovac A., Boniface A., Sova M., Gobec S., Blanot D. (2008) FEMS Microbiol. Rev. 32, 168–207 - PubMed
-
- Sauvage E., Kerff F., Terrak M., Ayala J. A., Charlier P. (2008) FEMS Microbiol. Rev. 32, 234–258 - PubMed
-
- Carballido-López R., Formstone A. (2007) Curr. Opin. Microbiol. 10, 611–616 - PubMed
-
- den Blaauwen T., de Pedro M. A., Nguyen-Distèche M., Ayala J. A. (2008) FEMS Microbiol. Rev. 32, 321–344 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

