PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells
- PMID: 20525692
- PMCID: PMC2915704
- DOI: 10.1074/jbc.M110.106278
PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells
Abstract
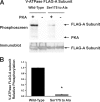
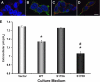
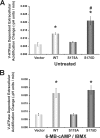
The vacuolar H(+)-ATPase (V-ATPase) is a major contributor to luminal acidification in epithelia of Wolffian duct origin. In both kidney-intercalated cells and epididymal clear cells, cAMP induces V-ATPase apical membrane accumulation, which is linked to proton secretion. We have shown previously that the A subunit in the cytoplasmic V(1) sector of the V-ATPase is phosphorylated by protein kinase A (PKA). Here we have identified by mass spectrometry and mutagenesis that Ser-175 is the major PKA phosphorylation site in the A subunit. Overexpression in HEK-293T cells of either a wild-type (WT) or phosphomimic Ser-175 to Asp (S175D) A subunit mutant caused increased acidification of HCO(3)(-)-containing culture medium compared with cells expressing vector alone or a PKA phosphorylation-deficient Ser-175 to Ala (S175A) mutant. Moreover, localization of the S175A A subunit mutant expressed in HEK-293T cells was more diffusely cytosolic than that of WT or S175D A subunit. Acute V-ATPase-mediated, bafilomycin-sensitive H(+) secretion was up-regulated by a specific PKA activator in HEK-293T cells expressing WT A subunit in HCO(3)(-)-free buffer. In cells expressing the S175D mutant, V-ATPase activity at the membrane was constitutively up-regulated and unresponsive to PKA activators, whereas cells expressing the S175A mutant had decreased V-ATPase activity that was unresponsive to PKA activation. Finally, Ser-175 was necessary for PKA-stimulated apical accumulation of the V-ATPase in a polarized rabbit cell line of collecting duct A-type intercalated cell characteristics (Clone C). In summary, these results indicate a novel mechanism for the regulation of V-ATPase localization and activity in kidney cells via direct PKA-dependent phosphorylation of the A subunit at Ser-175.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

