Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide
- PMID: 20525765
- PMCID: PMC2940696
- DOI: 10.1093/neuonc/noq044
Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide
Abstract
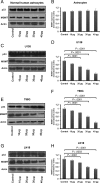
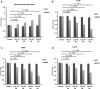
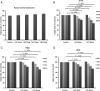
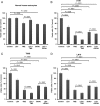
Antiepileptic drugs (AEDs) are frequently used to treat seizures in glioma patients. AEDs may have an unrecognized impact in modulating O(6)-methylguanine-DNA methyltransferase (MGMT), a DNA repair protein that has an important role in tumor cell resistance to alkylating agents. We report that levetiracetam (LEV) is the most potent MGMT inhibitor among several AEDs with diverse MGMT regulatory actions. In vitro, when used at concentrations within the human therapeutic range for seizure prophylaxis, LEV decreases MGMT protein and mRNA expression levels. Chromatin immunoprecipitation analysis reveals that LEV enhances p53 binding on the MGMT promoter by recruiting the mSin3A/histone deacetylase 1 (HDAC1) corepressor complex. However, LEV does not exert any MGMT inhibitory activity when the expression of either p53, mSin3A, or HDAC1 is abrogated. LEV inhibits malignant glioma cell proliferation and increases glioma cell sensitivity to the monofunctional alkylating agent temozolomide. In 4 newly diagnosed patients who had 2 craniotomies 7-14 days apart, prior to the initiation of any tumor-specific treatment, samples obtained before and after LEV treatment showed the inhibition of MGMT expression. Our results suggest that the choice of AED in patients with malignant gliomas may have an unrecognized impact in clinical practice and research trial design.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi:10.1056/NEJMoa043330. - DOI - PubMed
-
- Burton EC, Prados M. Malignant gliomas. Curr Treat Options Oncol. 2000;1(5):459–468. - PubMed
-
- Erickson LC, Bradley MO, Ducore JM, Ewig RA, Kohn KW. DNA crosslinking and cytotoxicity in normal and transformed human cells treated with antitumor nitrosoureas. Proc Natl Acad Sci USA. 1980;77(1):467–471. doi:10.1073/pnas.77.1.467. - DOI - PMC - PubMed
-
- Erickson LC, Laurent G, Sharkey NA, Kohn KW. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980;288(5792):727–729. doi:10.1038/288727a0. - DOI - PubMed
-
- Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462(2–3):83–100. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

