Engineered respiro-fermentative metabolism for the production of biofuels and biochemicals from fatty acid-rich feedstocks
- PMID: 20525863
- PMCID: PMC2916504
- DOI: 10.1128/AEM.00046-10
Engineered respiro-fermentative metabolism for the production of biofuels and biochemicals from fatty acid-rich feedstocks
Abstract
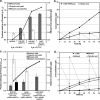
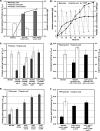
Although lignocellulosic sugars have been proposed as the primary feedstock for the biological production of renewable fuels and chemicals, the availability of fatty acid (FA)-rich feedstocks and recent progress in the development of oil-accumulating organisms make FAs an attractive alternative. In addition to their abundance, the metabolism of FAs is very efficient and could support product yields significantly higher than those obtained from lignocellulosic sugars. However, FAs are metabolized only under respiratory conditions, a metabolic mode that does not support the synthesis of fermentation products. In the work reported here we engineered several native and heterologous fermentative pathways to function in Escherichia coli under aerobic conditions, thus creating a respiro-fermentative metabolic mode that enables the efficient synthesis of fuels and chemicals from FAs. Representative biofuels (ethanol and butanol) and biochemicals (acetate, acetone, isopropanol, succinate, and propionate) were chosen as target products to illustrate the feasibility of the proposed platform. The yields of ethanol, acetate, and acetone in the engineered strains exceeded those reported in the literature for their production from sugars, and in the cases of ethanol and acetate they also surpassed the maximum theoretical values that can be achieved from lignocellulosic sugars. Butanol was produced at yields and titers that were between 2- and 3-fold higher than those reported for its production from sugars in previously engineered microorganisms. Moreover, our work demonstrates production of propionate, a compound previously thought to be synthesized only by propionibacteria, in E. coli. Finally, the synthesis of isopropanol and succinate was also demonstrated. The work reported here represents the first effort toward engineering microorganisms for the conversion of FAs to the aforementioned products.
Figures

Similar articles
-
The isc gene cluster expression ethanol tolerance associated improves its ethanol production by organic acids flux redirection in the ethanologenic Escherichia coli KO11 strain.World J Microbiol Biotechnol. 2019 Nov 20;35(12):189. doi: 10.1007/s11274-019-2769-8. World J Microbiol Biotechnol. 2019. PMID: 31748890
-
Biofuel production in Escherichia coli: the role of metabolic engineering and synthetic biology.Appl Microbiol Biotechnol. 2010 Mar;86(2):419-34. doi: 10.1007/s00253-010-2446-1. Epub 2010 Feb 9. Appl Microbiol Biotechnol. 2010. PMID: 20143230 Review.
-
Microbial production of fatty acid-derived fuels and chemicals.Curr Opin Biotechnol. 2013 Dec;24(6):1044-53. doi: 10.1016/j.copbio.2013.02.028. Epub 2013 Mar 28. Curr Opin Biotechnol. 2013. PMID: 23541503 Free PMC article. Review.
-
l-Rhamnose Metabolism in Clostridium beijerinckii Strain DSM 6423.Appl Environ Microbiol. 2019 Feb 20;85(5):e02656-18. doi: 10.1128/AEM.02656-18. Print 2019 Mar 1. Appl Environ Microbiol. 2019. PMID: 30578270 Free PMC article.
-
Metabolic engineering for production of biorenewable fuels and chemicals: contributions of synthetic biology.J Biomed Biotechnol. 2010;2010:761042. doi: 10.1155/2010/761042. Epub 2010 Apr 6. J Biomed Biotechnol. 2010. PMID: 20414363 Free PMC article. Review.
Cited by
-
Awakening sleeping beauty: production of propionic acid in Escherichia coli through the sbm operon requires the activity of a methylmalonyl-CoA epimerase.Microb Cell Fact. 2017 Jul 17;16(1):121. doi: 10.1186/s12934-017-0735-4. Microb Cell Fact. 2017. PMID: 28716098 Free PMC article.
-
Recent progress in synthetic biology for microbial production of C3-C10 alcohols.Front Microbiol. 2012 Jun 8;3:196. doi: 10.3389/fmicb.2012.00196. eCollection 2012. Front Microbiol. 2012. PMID: 22701113 Free PMC article.
-
Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli.Microb Cell Fact. 2019 Aug 6;18(1):130. doi: 10.1186/s12934-019-1177-y. Microb Cell Fact. 2019. PMID: 31387584 Free PMC article.
-
Reduction of hydrogen peroxide stress derived from fatty acid beta-oxidation improves fatty acid utilization in Escherichia coli.Appl Microbiol Biotechnol. 2014 Jan;98(2):629-39. doi: 10.1007/s00253-013-5327-6. Epub 2013 Oct 30. Appl Microbiol Biotechnol. 2014. PMID: 24169950 Free PMC article.
-
Engineering redox homeostasis to develop efficient alcohol-producing microbial cell factories.Microb Cell Fact. 2017 Jun 24;16(1):115. doi: 10.1186/s12934-017-0728-3. Microb Cell Fact. 2017. PMID: 28646866 Free PMC article. Review.
References
-
- Agreda, V. H., and J. R. Zoeller. 1993. Acetic acid and its derivatives. CRC Press, Boca Raton, FL.
-
- Atsumi, S., A. F. Cann, M. R. Connor, C. R. Shen, K. M. Smith, M. P. Brynildsen, K. J. Y. Chou, T. Hanai, and J. C. Liao. 2008. Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 10:305-311. - PubMed
-
- Bevan, M. W., and M. C. R. Franssen. 2006. Investing in green and white biotech. Nat. Biotechnol. 24:765-767. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

