Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice
- PMID: 20525877
- PMCID: PMC2930390
- DOI: 10.1152/ajpheart.00756.2009
Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice
Abstract
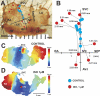
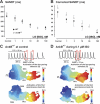
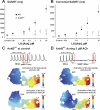
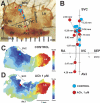
The mouse is widely used as a genetic platform to investigate the molecular mechanisms of sinoatrial node (SAN) pacemaking. Recently, it has been shown that isolated SAN cells from the ankyrin-B (AnkB)-deficient mice display severe pacemaking dysfunction similar to individuals harboring ankyrin 2 allele variants. However, these results have been limited to isolated SAN cells only and thus did not evaluate the functional anatomy of the widely distributed atrial pacemaker complex (e.g., the dynamic interaction of primary and subsidiary pacemakers). We studied pacemaker function in an intact mouse atrial preparation, which included the SAN, atrioventricular junction (AVJ), and both atria, excluding most of the septum. Optical mapping with a voltage-sensitive dye and CMOS camera ULTIMA-L was used to map spontaneous pacemaker activity with or without autonomic modulation in wild-type (WT) mice (n = 7) and in the AnkB heterozygous (AnkB(+/-); n = 9) mouse model of human SAN disease. In WT mice, isoproterenol accelerated the SAN rate (for 10 microM: from 325 + or - 19 to 510 + or - 33 beat/min, P < 0.01) and shifted the leading pacemaker site superiorly by 0.77 + or - 0.11 mm within the SAN. ACh decreased the SAN rate (from 333 + or - 26 to 96 + or - 22 beats/min, P < 0.01) and shifted the leading pacemaker either inferiorly within the SAN or abruptly toward the AVJ. After isoproterenol, AnkB(+/-) mice exhibited a larger beat-to-beat variability (SD of a cycle length: 13.4 + or - 3.6 vs. 2.5 + or - 0.8 ms, P < 0.01 vs. WT mice), disorganized shift of the leading pacemaker (2.04 + or - 0.37 mm, P < 0.05 vs. WT mice), and competing multiple pacemakers, resulting in beat-to-beat changes of the leading pacemaker location site between the SAN and AVJ regions. Notably, AnkB(+/-) mice also displayed a reduced sensitivity to ACh (rate slowing by 32 + or - 12% vs. 67 + or - 4%, P < 0.05, AnkB(+/-) vs. WT mice, respectively). In conclusion, AnkB dysfunction results in SAN abnormalities in an isolated mouse atria preparation. While AnkB dysfunction dramatically alters single SAN cell function, the mechanisms underlying cardiac automaticity are clearly complex, and phenotypes may be partially compensated by the dynamic interaction of cells within the pacemaker complex. These new findings highlight the importance of the functional anatomy of the entire atrial distributed pacemaker complex, including the SAN and AVJ, and clearly demonstrate the role of AnkB in cardiac automaticity.
Figures

References
-
- Adan V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 67: 1725–1732, 2003 - PubMed
-
- Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol 287: H1771–H1779, 2004 - PubMed
-
- Beau SL, Hand DE, Schuessler RB, Bromberg BI, Kwon B, Boineau JP, Saffitz JE. Relative densities of muscarinic cholinergic and beta-adrenergic receptors in the canine sinoatrial node and their relation to sites of pacemaker activity. Circ Res 77: 957–963, 1995 - PubMed
-
- Benditt D, Sakaguchi S, Goldstein M, Lurie K, Gornick C. Sinus node dysfunction: pathophysiology, clinical features, evaluation, and treatment. In: Cardiac Electrophysiology: From Cell to Bedside, edited by Zipes DP, Jalife J. Philadelphia, PA: Saunders, 1995, p. 1215–1247
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81: 1353–1392, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

