Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3+ regulatory T cells
- PMID: 20525891
- PMCID: PMC3575032
- DOI: 10.4049/jimmunol.0903846
Human T-lymphotropic virus type 1-induced CC chemokine ligand 22 maintains a high frequency of functional FoxP3+ regulatory T cells
Abstract
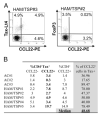
We recently reported that human T-lymphotropic virus type 1 (HTLV-1) infection is accompanied by a high frequency of CD4(+)FoxP3(+) cells in the circulation. In asymptomatic carriers of HTLV-1 and in patients with HTLV-1-associated inflammatory and malignant diseases, a high FoxP3(+) cell frequency correlated with inefficient cytotoxic T cell-mediated killing of HTLV-1-infected cells. In adult T cell leukemia/lymphoma (ATLL), the FoxP3(+) population was distinct from the leukemic T cell clones. However, the cause of the increase in FoxP3(+) cell frequency in HTLV-1 infection was unknown. In this study, we report that the plasma concentration of the chemokine CCL22 is abnormally high in HTLV-1-infected subjects and that the concentration is strongly correlated with the frequency of FoxP3(+) cells, which express the CCL22 receptor CCR4. Further, we show that CCL22 is produced by cells that express the HTLV-1 transactivator protein Tax, and that the increased CCL22 enhances the migration and survival of FoxP3(+) cells in vitro. Finally, we show that FoxP3(+) cells inhibit the proliferation of ex vivo, autologous leukemic clones from patients with ATLL. We conclude that HTLV-1-induced CCL22 causes the high frequency of FoxP3(+) cells observed in HTLV-1 infection; these FoxP3(+) cells may both retard the progression of ATLL and HTLV-1-associated inflammatory diseases and contribute to the immune suppression seen in HTLV-1 infection, especially in ATLL.
Figures






References
-
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. - PubMed
-
- Nagai M, Osame M. Human T-cell lymphotropic virus type I and neurological diseases. J. Neurovirol. 2003;9:228–235. - PubMed
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. - PubMed
-
- Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

