Estimating absolute risks in the presence of nonadherence: an application to a follow-up study with baseline randomization
- PMID: 20526200
- PMCID: PMC3315056
- DOI: 10.1097/EDE.0b013e3181df1b69
Estimating absolute risks in the presence of nonadherence: an application to a follow-up study with baseline randomization
Abstract
The intention-to-treat (ITT) analysis provides a valid test of the null hypothesis and naturally results in both absolute and relative measures of risk. However, this analytic approach may miss the occurrence of serious adverse effects that would have been detected under full adherence to the assigned treatment. Inverse probability weighting of marginal structural models has been used to adjust for nonadherence, but most studies have provided only relative measures of risk. In this study, we used inverse probability weighting to estimate both absolute and relative measures of risk of invasive breast cancer under full adherence to the assigned treatment in the Women's Health Initiative estrogen-plus-progestin trial. In contrast to an ITT hazard ratio (HR) of 1.25 (95% confidence interval [CI] = 1.01 to 1.54), the HR for 8-year continuous estrogen-plus-progestin use versus no use was 1.68 (1.24 to 2.28). The estimated risk difference (cases/100 women) at year 8 was 0.83 (-0.03 to 1.69) in the ITT analysis, compared with 1.44 (0.52 to 2.37) in the adherence-adjusted analysis. Results were robust across various dose-response models. We also compared the dynamic treatment regimen "take hormone therapy until certain adverse events become apparent, then stop taking hormone therapy" with no use (HR = 1.64; 95% CI = 1.24 to 2.18). The methods described here are also applicable to observational studies with time-varying treatments.
Figures

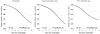
References
-
- Robins JM. Correction for non-compliance in equivalence trials. Stat Med. 1998;17(3):269–302. discussion 387-9. - PubMed
-
- Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. - PubMed
-
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. - PubMed
-
- Toh S, Hernán MA. Causal inference from longitudinal studies with baseline randomization. Int J Biostat. 2008;4(1):Article 22. Available at: http://www.bepress.com/ijb/vol4/iss1/22. - PMC - PubMed
-
- Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

