Shifting HIFs in osteoarthritis
- PMID: 20526316
- PMCID: PMC2917906
- DOI: 10.1038/nm0610-641
Shifting HIFs in osteoarthritis
Erratum in
- Nat Med. 2010 Jul;16(7):828
Abstract
There is no cure for osteoarthritis—the most common disease of the joints. By piecing together the molecular events that drive the progression of this debilitating disease, recent studies published in Nature Medicine put hypoxia-inducible factor-2α (HIF-2α) in the driver's seat, opening up new avenues for early detection and treatment (pages 678–686 and 687–693).
Figures
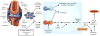
Comment on
-
Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction.Nat Med. 2010 Jun;16(6):687-93. doi: 10.1038/nm.2153. Epub 2010 May 23. Nat Med. 2010. PMID: 20495569
-
Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development.Nat Med. 2010 Jun;16(6):678-86. doi: 10.1038/nm.2146. Epub 2010 May 23. Nat Med. 2010. PMID: 20495570
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

