Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior
- PMID: 20526346
- PMCID: PMC2945246
- DOI: 10.1038/nmeth.1468
Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior
Erratum in
- Nat Methods. 2011 Feb;8(2):184
Abstract
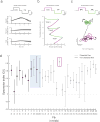
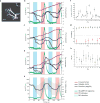
Drosophila melanogaster is a model organism rich in genetic tools to manipulate and identify neural circuits involved in specific behaviors. Here we present a technique for two-photon calcium imaging in the central brain of head-fixed Drosophila walking on an air-supported ball. The ball's motion is tracked at high resolution and can be treated as a proxy for the fly's own movements. We used the genetically encoded calcium sensor, GCaMP3.0, to record from important elements of the motion-processing pathway, the horizontal-system lobula plate tangential cells (LPTCs) in the fly optic lobe. We presented motion stimuli to the tethered fly and found that calcium transients in horizontal-system neurons correlated with robust optomotor behavior during walking. Our technique allows both behavior and physiology in identified neurons to be monitored in a genetic model organism with an extensive repertoire of walking behaviors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Walking modulates speed sensitivity in Drosophila motion vision.Curr Biol. 2010 Aug 24;20(16):1470-5. doi: 10.1016/j.cub.2010.06.072. Epub 2010 Jul 22. Curr Biol. 2010. PMID: 20655222 Free PMC article.
-
The diversity of lobula plate tangential cells (LPTCs) in the Drosophila motion vision system.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):139-148. doi: 10.1007/s00359-019-01380-y. Epub 2019 Nov 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31709462 Free PMC article.
-
Neural dynamics for landmark orientation and angular path integration.Nature. 2015 May 14;521(7551):186-91. doi: 10.1038/nature14446. Nature. 2015. PMID: 25971509 Free PMC article.
-
Fly motion vision.Annu Rev Neurosci. 2010;33:49-70. doi: 10.1146/annurev-neuro-060909-153155. Annu Rev Neurosci. 2010. PMID: 20225934 Review.
-
Optomotor-blind of Drosophila melanogaster: a neurogenetic approach to optic lobe development and optomotor behaviour.Comp Biochem Physiol A Physiol. 1995 Mar;110(3):185-202. doi: 10.1016/0300-9629(94)00159-q. Comp Biochem Physiol A Physiol. 1995. PMID: 7712063 Review.
Cited by
-
Competing visual flicker reveals attention-like rivalry in the fly brain.Front Integr Neurosci. 2012 Oct 19;6:96. doi: 10.3389/fnint.2012.00096. eCollection 2012. Front Integr Neurosci. 2012. PMID: 23091453 Free PMC article.
-
Mapping brain networks: Fish-bowl neuroscience.Nature. 2013 Jan 24;493(7433):466-8. doi: 10.1038/493466a. Nature. 2013. PMID: 23344340 No abstract available.
-
Long-term live imaging of the Drosophila adult midgut reveals real-time dynamics of division, differentiation and loss.Elife. 2018 Nov 14;7:e36248. doi: 10.7554/eLife.36248. Elife. 2018. PMID: 30427308 Free PMC article.
-
Generation of stable heading representations in diverse visual scenes.Nature. 2019 Dec;576(7785):126-131. doi: 10.1038/s41586-019-1767-1. Epub 2019 Nov 20. Nature. 2019. PMID: 31748750 Free PMC article.
-
The author file: Vivek Jayaraman.Nat Methods. 2010 Jul;7(7):483. doi: 10.1038/nmeth0710-483. Nat Methods. 2010. PMID: 20603910 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

