Dynamics of lamin-A processing following precursor accumulation
- PMID: 20526372
- PMCID: PMC2878336
- DOI: 10.1371/journal.pone.0010874
Dynamics of lamin-A processing following precursor accumulation
Abstract
Lamin A (LaA) is a component of the nuclear lamina, an intermediate filament meshwork that underlies the inner nuclear membrane (INM) of the nuclear envelope (NE). Newly synthesized prelamin A (PreA) undergoes extensive processing involving C-terminal farnesylation followed by proteolysis yielding non-farnesylated mature lamin A. Different inhibitors of these processing events are currently used therapeutically. Hutchinson-Gilford Progeria Syndrome (HGPS) is most commonly caused by mutations leading to an accumulation of a farnesylated LaA isoform, prompting a clinical trial using farnesyltransferase inhibitors (FTI) to reduce this modification. At therapeutic levels, HIV protease inhibitors (PI) can unexpectedly inhibit the final processing step in PreA maturation. We have examined the dynamics of LaA processing and associated cellular effects during PI or FTI treatment and following inhibitor washout. While PI reversibility was rapid, with respect to both LaA maturation and associated cellular phenotype, recovery from FTI treatment was more gradual. FTI reversibility is influenced by both cell type and rate of proliferation. These results suggest a less static lamin network than has previously been observed.
Conflict of interest statement
Figures

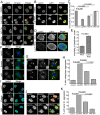
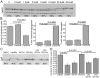



Similar articles
-
Blocking protein farnesylation improves nuclear shape abnormalities in keratinocytes of mice expressing the prelamin A variant in Hutchinson-Gilford progeria syndrome.Nucleus. 2010 Sep-Oct;1(5):432-9. doi: 10.4161/nucl.1.5.12972. Nucleus. 2010. PMID: 21326826 Free PMC article.
-
Blocking farnesylation of the prelamin A variant in Hutchinson-Gilford progeria syndrome alters the distribution of A-type lamins.Nucleus. 2012 Sep-Oct;3(5):452-62. doi: 10.4161/nucl.21675. Epub 2012 Aug 16. Nucleus. 2012. PMID: 22895092 Free PMC article.
-
Prelamin A farnesylation and progeroid syndromes.J Biol Chem. 2006 Dec 29;281(52):39741-5. doi: 10.1074/jbc.R600033200. Epub 2006 Nov 7. J Biol Chem. 2006. PMID: 17090536 Review.
-
Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes.Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12873-8. doi: 10.1073/pnas.0505767102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129834 Free PMC article.
-
Lamin A, farnesylation and aging.Exp Cell Res. 2012 Jan 1;318(1):1-7. doi: 10.1016/j.yexcr.2011.08.009. Epub 2011 Aug 16. Exp Cell Res. 2012. PMID: 21871450 Free PMC article. Review.
Cited by
-
Mechanisms of A-Type Lamin Targeting to Nuclear Ruptures Are Disrupted in LMNA- and BANF1-Associated Progerias.Cells. 2022 Mar 2;11(5):865. doi: 10.3390/cells11050865. Cells. 2022. PMID: 35269487 Free PMC article.
-
Post-Translational Modification of Lamins: Mechanisms and Functions.Front Cell Dev Biol. 2022 May 17;10:864191. doi: 10.3389/fcell.2022.864191. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656549 Free PMC article. Review.
-
Subcellular localization of SREBP1 depends on its interaction with the C-terminal region of wild-type and disease related A-type lamins.Exp Cell Res. 2011 Dec 10;317(20):2800-13. doi: 10.1016/j.yexcr.2011.09.012. Epub 2011 Oct 4. Exp Cell Res. 2011. PMID: 21993218 Free PMC article.
-
Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson-Gilford progeria fibroblasts.Oncotarget. 2017 Jul 18;8(39):64809-64826. doi: 10.18632/oncotarget.19363. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029393 Free PMC article.
-
Structure and stability of the lamin A tail domain and HGPS mutant.J Struct Biol. 2011 Sep;175(3):425-33. doi: 10.1016/j.jsb.2011.05.015. Epub 2011 May 24. J Struct Biol. 2011. PMID: 21635954 Free PMC article.
References
-
- Burke B, Stewart CL. Life at the edge: the nuclear envelope and human disease. Nat Rev Mol Cell Biol. 2002;3:575–585. - PubMed
-
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. - PubMed
-
- Gerace L, Comeau C, Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. - PubMed
-
- Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

