Dynamics of lamin-A processing following precursor accumulation
- PMID: 20526372
- PMCID: PMC2878336
- DOI: 10.1371/journal.pone.0010874
Dynamics of lamin-A processing following precursor accumulation
Abstract
Lamin A (LaA) is a component of the nuclear lamina, an intermediate filament meshwork that underlies the inner nuclear membrane (INM) of the nuclear envelope (NE). Newly synthesized prelamin A (PreA) undergoes extensive processing involving C-terminal farnesylation followed by proteolysis yielding non-farnesylated mature lamin A. Different inhibitors of these processing events are currently used therapeutically. Hutchinson-Gilford Progeria Syndrome (HGPS) is most commonly caused by mutations leading to an accumulation of a farnesylated LaA isoform, prompting a clinical trial using farnesyltransferase inhibitors (FTI) to reduce this modification. At therapeutic levels, HIV protease inhibitors (PI) can unexpectedly inhibit the final processing step in PreA maturation. We have examined the dynamics of LaA processing and associated cellular effects during PI or FTI treatment and following inhibitor washout. While PI reversibility was rapid, with respect to both LaA maturation and associated cellular phenotype, recovery from FTI treatment was more gradual. FTI reversibility is influenced by both cell type and rate of proliferation. These results suggest a less static lamin network than has previously been observed.
Conflict of interest statement
Figures

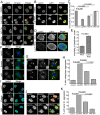
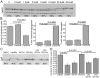



References
-
- Burke B, Stewart CL. Life at the edge: the nuclear envelope and human disease. Nat Rev Mol Cell Biol. 2002;3:575–585. - PubMed
-
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. - PubMed
-
- Gerace L, Comeau C, Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. - PubMed
-
- Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

