Histone Deacetylase Inhibitor M344 Inhibits Cell Proliferation and Induces Apoptosis in Human THP-1 Leukemia Cells
- PMID: 20526416
- PMCID: PMC2880493
- DOI: 10.5099/aj090400352
Histone Deacetylase Inhibitor M344 Inhibits Cell Proliferation and Induces Apoptosis in Human THP-1 Leukemia Cells
Abstract
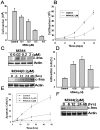
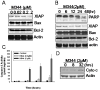
Histone acetylation plays an important role in the silencing and activation of genes involved in tumoregenesis. Trichostatin A, originally identified as an anti-fungal drug, is a potent inhibitor of histone deacetylase (HDAC) with potential anti-tumor activity. In this study, we investigated the effect of M344, an amide analogues of trichostatin A, on the growth and differentiation of THP-1 human leukemia cells. We showed that at low doses, (< 0.2 muM), M344 could inhibit the growth of THP-1 cells at G1 phase in vitro with low cytotoxic effect. Low dose of M344 exerted some differentiating effect on THP-1 cells as judged by the expression of c-fms proto-oncogene (M-CSF receptor) and appearance of adherent cells. Growth arrest induced by M344 is associated with increased levels of cyclin-dependent protein kinase inhibitor p21 and cyclin E, in agreement with G1 phase arrest. At higher doses (2 muM), M344 could induce THP-1 cells to undergo apoptosis, which was associated with the cleavage of PARP, cytochrome c release and activation of both caspases-8, -9, followed by the activation of caspase-3. In addition, M344 could increase the levels of pro-apoptotic protein Bax but decreased the levels of anti-apoptotic protein XIAP. M344 is a potent activator of NF-kappaB transcription factor. RT-PCR assay showed that the M344 could transiently increase IL-1 expression yet markedly decreased TNF-alpha expression. Our results show that M344 is a potent growth inhibitor and inducer of apoptosis in human leukemia cells and suggest potential therapeutic strategies of HDAC inhibitors for patients with leukemias.
Figures


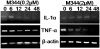
Similar articles
-
Selective histonedeacetylase inhibitor M344 intervenes in HIV-1 latency through increasing histone acetylation and activation of NF-kappaB.PLoS One. 2012;7(11):e48832. doi: 10.1371/journal.pone.0048832. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23166597 Free PMC article.
-
M344 is a novel synthesized histone deacetylase inhibitor that induces growth inhibition, cell cycle arrest, and apoptosis in human endometrial cancer and ovarian cancer cells.Gynecol Oncol. 2006 Apr;101(1):108-13. doi: 10.1016/j.ygyno.2005.09.044. Epub 2005 Nov 2. Gynecol Oncol. 2006. Retraction in: Gynecol Oncol. 2013 Apr;129(1):270. doi: 10.1016/j.ygyno.2012.09.022. PMID: 16263156 Retracted.
-
HDAC inhibitor M344 suppresses MCF-7 breast cancer cell proliferation.Biomed Pharmacother. 2012 Apr;66(3):232-6. doi: 10.1016/j.biopha.2011.06.007. Epub 2011 Aug 27. Biomed Pharmacother. 2012. PMID: 22436652
-
The histone deacetylase inhibitor suberic bishydroxamate: a potential sensitizer of melanoma to TNF-related apoptosis-inducing ligand (TRAIL) induced apoptosis.Biochem Pharmacol. 2003 Oct 15;66(8):1537-45. doi: 10.1016/s0006-2952(03)00509-4. Biochem Pharmacol. 2003. PMID: 14555232 Review.
-
Copper metabolism in cell death and autophagy.Autophagy. 2023 Aug;19(8):2175-2195. doi: 10.1080/15548627.2023.2200554. Epub 2023 Apr 16. Autophagy. 2023. PMID: 37055935 Free PMC article. Review.
Cited by
-
Molecular targeting of prostate cancer cells by a triple drug combination down-regulates integrin driven adhesion processes, delays cell cycle progression and interferes with the cdk-cyclin axis.BMC Cancer. 2011 Aug 25;11:375. doi: 10.1186/1471-2407-11-375. BMC Cancer. 2011. PMID: 21867506 Free PMC article.
-
Expression pattern of class I histone deacetylases in vulvar intraepithelial neoplasia and vulvar cancer: a tissue microarray study.BMC Cancer. 2011 Oct 26;11:463. doi: 10.1186/1471-2407-11-463. BMC Cancer. 2011. PMID: 22029821 Free PMC article.
-
HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase pi in prostate carcinoma cells.Mol Cancer Res. 2011 Jun;9(6):733-45. doi: 10.1158/1541-7786.MCR-10-0505. Epub 2011 May 26. Mol Cancer Res. 2011. PMID: 21622623 Free PMC article.
-
The Differential Antitumor Activity of 5-Aza-2'-deoxycytidine in Prostate Cancer DU145, 22RV1, and LNCaP Cells.J Cancer. 2021 Jul 25;12(18):5593-5604. doi: 10.7150/jca.56709. eCollection 2021. J Cancer. 2021. PMID: 34405020 Free PMC article.
-
Selective histonedeacetylase inhibitor M344 intervenes in HIV-1 latency through increasing histone acetylation and activation of NF-kappaB.PLoS One. 2012;7(11):e48832. doi: 10.1371/journal.pone.0048832. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23166597 Free PMC article.
References
-
- Marmorstein R. Structural and chemical basis of histone acetylation. Novartis Found Symp. 2004;259:78–98. discussion 98–101, 163–9. - PubMed
-
- Wang S, et al. Transcription regulation by histone deacetylases. Novartis Found Symp. 2004;259:238–45. discussion 245–8, 285–8. - PubMed
-
- Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20(8):615–26. - PubMed
-
- Ito K, I, Adcock M. Histone acetylation and histone deacetylation. Mol Biotechnol. 2002;20(1):99–106. - PubMed
-
- Chambers AE, et al. Histone acetylation-mediated regulation of genes in leukaemic cells. Eur J Cancer. 2003;39(8):1165–75. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
