Asymmetric synthesis of D-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy)acetaldehyde
- PMID: 20527744
- PMCID: PMC2892910
- DOI: 10.1021/jo100707d
Asymmetric synthesis of D-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy)acetaldehyde
Abstract
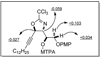
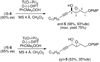
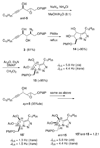
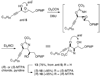
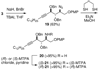
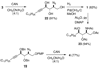
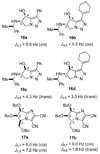
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol (12)-catalyzed alkynylation of unsaturated aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN(3)/NH(4)Cl. Deprotection and reduction of the resulting acyclic azide 3 then gave 1. Alkyne-azide 3 was subjected to an intramolecular click reaction, generating a bicyclic triazole, which was found to have unexpected vicinal coupling constants. Application of the advanced Mosher method verified the configurations of the three contiguous stereogenic centers of 1. An alkynyl azide analogue of 1, which may be useful as a glycosyl acceptor in the synthesis of alpha-galactosylceramide derivatives, was also readily prepared by this route.
Figures





Similar articles
-
Preparation of anti-vicinal amino alcohols: asymmetric synthesis of D-erythro-sphinganine, (+)-spisulosine, and D-ribo-phytosphingosine.J Org Chem. 2013 Jul 19;78(14):7223-33. doi: 10.1021/jo401211j. Epub 2013 Jul 3. J Org Chem. 2013. PMID: 23795558 Free PMC article.
-
A versatile synthesis of αGalCer and its analogues exploiting a cyclic carbonate as phytosphingosine 3,4-diol protecting group.Carbohydr Res. 2019 Jan 15;472:50-57. doi: 10.1016/j.carres.2018.11.005. Epub 2018 Nov 15. Carbohydr Res. 2019. PMID: 30471510
-
Dihydroxylation of 2-vinylaziridine: efficient synthesis of D-ribo-phytosphingosine.Chem Commun (Camb). 2007 Jan 7;(1):79-81. doi: 10.1039/b612740a. Epub 2006 Nov 17. Chem Commun (Camb). 2007. PMID: 17279267
-
Cu-catalyzed azide-alkyne cycloaddition.Chem Rev. 2008 Aug;108(8):2952-3015. doi: 10.1021/cr0783479. Chem Rev. 2008. PMID: 18698735 Review. No abstract available.
-
Synthesis of Porphyrin, Chlorin and Phthalocyanine Derivatives by Azide-Alkyne Click Chemistry.Curr Med Chem. 2015;22(28):3217-54. doi: 10.2174/0929867322666150716115832. Curr Med Chem. 2015. PMID: 26179994 Review.
Cited by
-
Preparation of anti-vicinal amino alcohols: asymmetric synthesis of D-erythro-sphinganine, (+)-spisulosine, and D-ribo-phytosphingosine.J Org Chem. 2013 Jul 19;78(14):7223-33. doi: 10.1021/jo401211j. Epub 2013 Jul 3. J Org Chem. 2013. PMID: 23795558 Free PMC article.
-
Total synthesis of α-1C-galactosylceramide, an immunostimulatory C-glycosphingolipid, and confirmation of the stereochemistry in the first-generation synthesis.J Org Chem. 2011 Nov 4;76(21):8588-98. doi: 10.1021/jo201450s. Epub 2011 Oct 4. J Org Chem. 2011. PMID: 21958232 Free PMC article.
-
Recent Progress in the Diverse Synthetic Approaches to Phytosphingosine.Top Curr Chem (Cham). 2025 Aug 31;383(3):35. doi: 10.1007/s41061-025-00518-8. Top Curr Chem (Cham). 2025. PMID: 40886224 Review.
-
Diastereoselective Synthesis of the HIV Protease Inhibitor Darunavir and Related Derivatives via a Titanium Tetrachloride-Mediated Asymmetric Glycolate Aldol Addition Reaction.J Org Chem. 2024 Jul 5;89(13):9569-9585. doi: 10.1021/acs.joc.4c01057. Epub 2024 Jun 25. J Org Chem. 2024. PMID: 38916048 Free PMC article.
References
-
- Veerman ECI, Valentijn-Benz M, van't Hof W, Nazmi K, van Marle J, Amerongen AVN. Biol. Chem. 2010;391:65–71. - PubMed
-
- Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. J. Biol. Chem. 1997;272:32566–32572. - PubMed
- Skrzypek MS, Nagiec MM, Lester RL, Dickson RC. J. Bacteriol. 1999;181:1134–1140. - PMC - PubMed
- Meier KD, Deloche O, Kajiwara K, Funato K, Riezman H. Mol. Biol. Cell. 2006;17:1164–1175. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

