Asymmetric synthesis of D-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy)acetaldehyde
- PMID: 20527744
- PMCID: PMC2892910
- DOI: 10.1021/jo100707d
Asymmetric synthesis of D-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy)acetaldehyde
Abstract
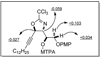
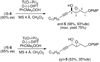
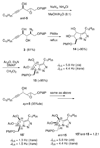
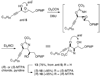
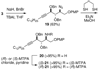
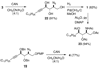
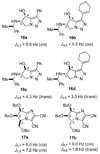
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol (12)-catalyzed alkynylation of unsaturated aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN(3)/NH(4)Cl. Deprotection and reduction of the resulting acyclic azide 3 then gave 1. Alkyne-azide 3 was subjected to an intramolecular click reaction, generating a bicyclic triazole, which was found to have unexpected vicinal coupling constants. Application of the advanced Mosher method verified the configurations of the three contiguous stereogenic centers of 1. An alkynyl azide analogue of 1, which may be useful as a glycosyl acceptor in the synthesis of alpha-galactosylceramide derivatives, was also readily prepared by this route.
Figures





References
-
- Veerman ECI, Valentijn-Benz M, van't Hof W, Nazmi K, van Marle J, Amerongen AVN. Biol. Chem. 2010;391:65–71. - PubMed
-
- Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. J. Biol. Chem. 1997;272:32566–32572. - PubMed
- Skrzypek MS, Nagiec MM, Lester RL, Dickson RC. J. Bacteriol. 1999;181:1134–1140. - PMC - PubMed
- Meier KD, Deloche O, Kajiwara K, Funato K, Riezman H. Mol. Biol. Cell. 2006;17:1164–1175. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

