Solid-phase synthesis of N-substituted pyrrolidinone-tethered N-substituted piperidines via Ugi reaction
- PMID: 20527770
- PMCID: PMC2917892
- DOI: 10.1021/cc100054u
Solid-phase synthesis of N-substituted pyrrolidinone-tethered N-substituted piperidines via Ugi reaction
Abstract
Starting with resin-bound glutamic acid, an efficient synthesis of N-substituted pyrrolidinones is described, using the Ugi four-component reaction (U-4CR). The same methodology is employed to produce N-substituted pyrrolidinone tethered N-substituted piperidines.
Figures
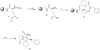
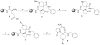
References
-
- Zhu J, Bienaymé H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2005. and references therein.
- Simila STM, Martin SF. Tet Lett. 2008;49:450. - PMC - PubMed
- El Kaim L, Grimaud L. Tetrahedron. 2009;65:2153.
- Hulme C, Dietrich J. Molecular Diversity. 2009;13:195. - PubMed
- Domling A. Chem Rev. 2006;106:17. - PubMed
-
- Dömling A. Chem Rev. 2006;106:17–89. - PubMed
- Marcaccini S, Torroba T. Nat Protoc. 2007;2:632–639. - PubMed
- Dömling A, Ugi I. Angew Chem, Int Ed. 2000;39:3168–3210. - PubMed
- Ugi I, Werner B, Dömling A. Molecules. 2003;8:53–56.
- Main M, Snaith JS, Meloni MM, Jauregui M, Sykes D, Faulkner S, Kenwright AM. Chem Commun. 2008:5212–5214. - PubMed
-
- Pulici M, Quartieri F, Felder ER. J Comb Chem. 2005;7:463–473. - PubMed
- Hoel AML, Nielson J. Tetrahedron Lett. 1999;40:3941–3944.
- Tempest RA, Brown SD, Armstrong RW. Angewandte Chemie, International Edition in English. 1996;35:640.
- Lin Q, O’Neill JC, Blackwell HE. Organic Letters. 2005;7:4455. - PubMed
- Stocker AM, Keating TA, Tempest PA, Armstrong RW. Tetrahedron Letters. 1996. p. 1149.
- Golebiowski A, Jozwik J, Klopfenstein SR, Colson A-O, Grieb AL, Russell AF, Rastogi VL, Diven CF, Portlock DE, Chen JJ. Journal of Combinatorial Chemistry. 2002;4:584. - PubMed
- Golebiowski A, Jozwik J, Klopfenstein SR, Colson A-O, Grieb AL, Russell AF, Rastogi VL, Diven CF, Portlock DE, Chen JJ. Journal of Combinatorial Chemistry. 2002;4:584–590. - PubMed
- Suda A, Ohta A, Sudoh M, Tsukuda T, Shimma N. Heterocycles. 2001;55:1023.
- Sutherlin DP, Stark TM, Hughes R, Armstrong RW. Journal of Organic Chemistry. 1996;61:8350. - PubMed
- Stocker AM, Keating TA, Tempest PA, Armstrong RW. Tetrahedron Letters. 1996;37:1149.
-
- Hanessian S, Yun H, Hou Y, Tintelnot-Blomley M. J Org Chem. 2005;70:6746–6756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

