A formal synthesis of the C1-C9 fragment of amphidinolide C employing the Tamaru reaction
- PMID: 20527780
- PMCID: PMC2896498
- DOI: 10.1021/ol100959a
A formal synthesis of the C1-C9 fragment of amphidinolide C employing the Tamaru reaction
Abstract
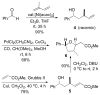
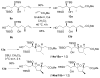
Homoallylation of aldehydes with isoprene and triethylborane catalyzed by Ni(acac)(2) gave hydroxyalkenes in good yield with excellent regio- and stereoselectivity. Cross metathesis of the hydroxyalkenes with methyl acrylate using second-generation Grubbs catalyst and copper(I) iodide afforded alpha,beta-unsaturated esters, which underwent cyclization in the presence of DBU to produce tetrahydrofurans with the correct relative configuration for the C1-C9 fragment of amphidinolides C, C2, and F.
Figures

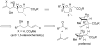




Similar articles
-
Synthesis of the C(18)-C(34) fragment of amphidinolide C and the C(18)-C(29) fragment of amphidinolide F.Org Lett. 2010 Nov 19;12(22):5326-9. doi: 10.1021/ol102345v. Epub 2010 Oct 28. Org Lett. 2010. PMID: 21028791 Free PMC article.
-
Ni-catalyzed homoallylation of polyhydroxy n,o-acetals with conjugated dienes promoted by triethylborane.Molecules. 2014 Jul 2;19(7):9288-306. doi: 10.3390/molecules19079288. Molecules. 2014. PMID: 24991760 Free PMC article.
-
Cationic rare-earth-metal half-sandwich complexes for the living trans-1,4-isoprene polymerization.Angew Chem Int Ed Engl. 2008;47(4):775-8. doi: 10.1002/anie.200703514. Angew Chem Int Ed Engl. 2008. PMID: 18080266 No abstract available.
-
Selective hydroformylation-hydrogenation tandem reaction of isoprene to 3-methylpentanal.Dalton Trans. 2011 Nov 28;40(44):11742-7. doi: 10.1039/c1dt11292a. Epub 2011 Sep 29. Dalton Trans. 2011. PMID: 21960209
-
A review of stereochemical implications in the generation of secondary organic aerosol from isoprene oxidation.Environ Sci Process Impacts. 2016 Nov 9;18(11):1369-1380. doi: 10.1039/c6em00354k. Environ Sci Process Impacts. 2016. PMID: 27762408 Review.
Cited by
-
Synthesis of the C(18)-C(34) fragment of amphidinolide C and the C(18)-C(29) fragment of amphidinolide F.Org Lett. 2010 Nov 19;12(22):5326-9. doi: 10.1021/ol102345v. Epub 2010 Oct 28. Org Lett. 2010. PMID: 21028791 Free PMC article.
-
Exploiting hidden symmetry in natural products: total syntheses of amphidinolides C and F.J Am Chem Soc. 2013 Jul 24;135(29):10792-803. doi: 10.1021/ja404796n. Epub 2013 Jul 11. J Am Chem Soc. 2013. PMID: 23845005 Free PMC article.
-
Synthetic Studies on Amphidinolide F: Exploration of Macrocycle Construction by Intramolecular Stille Coupling.Org Lett. 2022 Oct 21;24(41):7600-7604. doi: 10.1021/acs.orglett.2c03045. Epub 2022 Oct 12. Org Lett. 2022. PMID: 36223230 Free PMC article.
-
Studies toward the Synthesis of Amphidinolide C1: Stereoselective Construction of the C(1)-C(15) Segment.Org Lett. 2020 Dec 4;22(23):9174-9177. doi: 10.1021/acs.orglett.0c03134. Epub 2020 Nov 12. Org Lett. 2020. PMID: 33180502 Free PMC article.
-
Convergent Synthesis of the C1-C29 Framework of Amphidinolide F.J Org Chem. 2022 Jun 17;87(12):8126-8141. doi: 10.1021/acs.joc.2c00850. Epub 2022 Jun 8. J Org Chem. 2022. PMID: 35675580 Free PMC article.
References
-
- Kobayashi J, Ishibashi M, Walchli MR, Nakamura H, Hirata Y, Sasaki T, Ohizumi Y. J Am Chem Soc. 1988;110:490.
- Kubota T, Tsuda M, Kobayashi J. Org Lett. 2001;3:1363. - PubMed
-
- Bates RH, Shotwell JB, Roush WR. Org Lett. 2008;10:4343. - PMC - PubMed
- Shotwell JB, Roush WR. Org Lett. 2004;6:3865. - PubMed
- Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2003;59:1613.
- Armstrong A, Pyrkotis C. Tetrahedron Lett. 2009;50:3325.
- Mohapatra DK, Dasari PK, Rahaman H, Pal R. Tetrahedron Lett. 2009;50:6276.
- Mohapatra DK, Rahaman H, Chorghade MS, Gurjar MK. Synlett. 2007;4:567.
- Mahapatra S, Carter RG. Org Biomol Chem. 2009;7:4582. - PMC - PubMed
-
- Haney ME, Hoehn MM. Antimicrob Agents Chemother. 1968;7:349. - PubMed
-
- Tsuda M, Endo T, Kobayashi J. J Org Chem. 2000;65:1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

