Real-time bioluminescence imaging of glycans on live cells
- PMID: 20527879
- PMCID: PMC2890245
- DOI: 10.1021/ja101766r
Real-time bioluminescence imaging of glycans on live cells
Abstract
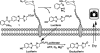
Cell-surface glycans are attractive targets for molecule imaging due to their reflection of cellular processes associated with development and disease progression. In this paper, we describe the design, synthesis, and biological application of a new phosphine probe for real-time imaging of cell-surface glycans using bioluminescence. To accomplish this goal, we took advantage of the bioorthogonal chemical reporter technique. This strategy uses a two-step labeling procedure in which an unnatural sugar analogue containing a functional handle is (1) incorporated into sugar-bearing proteins via the cell's own biosynthetic machinery and then (2) detected with an exogenously added probe. We designed phosphine-luciferin reagent 1 to activate bioluminescence in response to Staudinger ligation with azide-labeled glycans. We chose to use a phosphine probe because, despite their slow reaction kinetics, they remain the best-performing reagents for tagging azidosugars in mice. Given the sensitivity and negligible background provided by bioluminescence imaging (BLI), we reasoned that 1 might be able to overcome some of the limitations encountered with fluorescent phosphine probes. In this work, we synthesized the first phosphine-luciferin probe for use in real-time BLI and demonstrated that azide-labeled cell-surface glycans can be imaged with 1 using concentrations as low as single digit nanomolar and times as little as 5 min, a feat that cannot be matched by any previous fluorescent phosphine probes. Even though we have only demonstrated its use in visualizing glycans, it can be envisioned that this probe could also be used for bioluminescence imaging of any azide-containing biomolecule, such as proteins and lipids, since azides have been previously incorporated into these molecules. The phosphine-luciferin probe is therefore poised for many applications in real-time imaging in cells and whole animals. These studies are currently in progress in our laboratory.
Figures


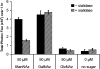

Similar articles
-
Synthesis of phosphine and antibody-azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach.Bioconjug Chem. 2011 Oct 19;22(10):2072-81. doi: 10.1021/bc200298v. Epub 2011 Sep 9. Bioconjug Chem. 2011. PMID: 21854058
-
Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation.Nat Protoc. 2007;2(11):2930-44. doi: 10.1038/nprot.2007.422. Nat Protoc. 2007. PMID: 18007630
-
Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones.J Am Chem Soc. 2010 Mar 24;132(11):3688-90. doi: 10.1021/ja100014q. J Am Chem Soc. 2010. PMID: 20187640 Free PMC article.
-
Staudinger ligation as a method for bioconjugation.Angew Chem Int Ed Engl. 2011 Sep 12;50(38):8806-27. doi: 10.1002/anie.201008102. Epub 2011 Sep 2. Angew Chem Int Ed Engl. 2011. PMID: 21887733 Review.
-
In Vivo Molecular Bioluminescence Imaging: New Tools and Applications.Trends Biotechnol. 2017 Jul;35(7):640-652. doi: 10.1016/j.tibtech.2017.03.012. Epub 2017 May 10. Trends Biotechnol. 2017. PMID: 28501458 Review.
Cited by
-
Design strategies for bioorthogonal smart probes.Org Biomol Chem. 2014 Dec 14;12(46):9307-20. doi: 10.1039/c4ob01632g. Epub 2014 Oct 15. Org Biomol Chem. 2014. PMID: 25315039 Free PMC article. Review.
-
The C-Terminal O-S Acyl Shift Pathway under Acidic Condition to Propose Peptide-Thioesters.Molecules. 2016 Nov 17;21(11):1559. doi: 10.3390/molecules21111559. Molecules. 2016. PMID: 27869694 Free PMC article.
-
Click chemistry and drug delivery: A bird's-eye view.Acta Pharm Sin B. 2023 May;13(5):1990-2016. doi: 10.1016/j.apsb.2022.10.015. Epub 2022 Oct 22. Acta Pharm Sin B. 2023. PMID: 37250163 Free PMC article. Review.
-
A biocompatible "split luciferin" reaction and its application for non-invasive bioluminescent imaging of protease activity in living animals.Curr Protoc Chem Biol. 2014 Sep 9;6(3):169-189. doi: 10.1002/9780470559277.ch140047. Curr Protoc Chem Biol. 2014. PMID: 25205565 Free PMC article.
-
Bioorthogonal Ligations and Cleavages in Chemical Biology.ChemistryOpen. 2020 Aug 14;9(8):835-853. doi: 10.1002/open.202000128. eCollection 2020 Aug. ChemistryOpen. 2020. PMID: 32817809 Free PMC article. Review.
References
-
- Varki A.; Cummings R. D.; Esko J. D.; Freeze H. H.; Stanley P.; Bertozzi C. R.; Hart G. W.; Etzler M. E.. Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

