[2+2] cycloadditions by oxidative visible light photocatalysis
- PMID: 20527886
- PMCID: PMC2892825
- DOI: 10.1021/ja103934y
[2+2] cycloadditions by oxidative visible light photocatalysis
Abstract
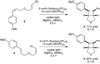
Photochemical reactions are remarkable for their ability to easily assemble cyclobutanes and other strained ring systems that are difficult to construct using other conventional synthetic methods. We have previously shown that Ru(bpy)(3)(2+) is an efficient photocatalyst that promotes the [2+2] cycloadditions of electron-deficient olefins with visible light. Here, we show that Ru(bpy)(3)(2+) is also an effective photocatalyst for the [2+2] cycloaddition of electron-rich olefins. This transformation is enabled by the versatile photoelectrochemical properties of Ru(bpy)(3)(2+), which enables either one-electron reduction or one-electron oxidation of interesting organic substrates under appropriate conditions.
Figures
References
-
- Hansen TV, Stenstrøm Y. “Naturally Occurring Cyclobutanes.”. In: Hudlicky T, editor. Organic Synthesis: Theory and Applications. Vol 5. Oxford, U.K: Elsevier; 2001. pp. 1–38.
- Dembitsky VM. J. Nat. Med. 2008;62:1–33. - PubMed
-
-
For recent reviews, see: Crimmins MT. Chem. Rev. 1988;88:1453–1473. Bach T. Synthesis. 1998:683–708. Lee-Ruff E, Mladenova G. Chem. Rev. 2003;103:1449–1483. Hoffman N. Chem. Rev. 2008;108:1052–1103.
-
-
- Esser P, Pohlmann B, Scharf H-D. Angew. Chem. Int. Ed. Engl. 1994;33:2009–2023.
-
- Nicewicz D, MacMillan DWC. Science. 2008;322:70–80. - PMC - PubMed
- Nagib DA, Scott ME, MacMillan DWC. J. Am. Chem. Soc. 2009;131:10875–10877. - PMC - PubMed
- Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756–8757. - PubMed
- Tucker JW, Narayanam JMR, Krabbe SW, Stephenson CRJ. Org. Lett. 2010;12:368–371. - PubMed
- Condie AG, González-Gómez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464–1465. - PubMed
- Koike T, Akita M. Chem. Lett. 2009;38:166–167.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources