Low concentrations of anti-Aβ antibodies generated in Tg2576 mice by DNA epitope vaccine fused with 3C3d molecular adjuvant do not affect AD pathology
- PMID: 20528468
- PMCID: PMC2978548
- DOI: 10.1089/hum.2009.219
Low concentrations of anti-Aβ antibodies generated in Tg2576 mice by DNA epitope vaccine fused with 3C3d molecular adjuvant do not affect AD pathology
Abstract
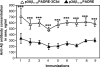
It has been demonstrated that an active vaccination strategy with protein- or DNA-based epitope vaccines composed of the immunodominant self B cell epitope of amyloid-β₄₂ (Aβ₄₂) and a non-self T helper (Th) cell epitope is an immunotherapeutic approach to preventing or treating Alzheimer's disease (AD). As a DNA-based epitope vaccine, we used a plasmid encoding three copies of Aβ(1-11) and Th cell epitope, PADRE (p3Aβ(1-11)-PADRE). We have previously reported that three copies of component of complement C3d (3C3d) acts as a molecular adjuvant significantly enhancing immune responses in wild-type mice of the H2(b) haplotype immunized with p3Aβ(1-11)-PADRE. Here, we tested the efficacy of p3Aβ(1-11)-PADRE and the same vaccine fused with 3C3d (p3Aβ(1-11)-PADRE-3C3d) in a transgenic (Tg) mouse model of AD (Tg2576) of the H2(bxs) immune haplotype. The overall responses to both vaccines were very weak in Tg2576 mice despite the fact that the 3C3d molecular adjuvant significantly enhanced the anti-Aβ response to 3Aβ(1-11)-PADRE. Importantly, generation of low antibody responses was associated with the strain of amyloid precursor protein Tg mice rather than with a molecular adjuvant, as a p3Aβ(1-11)-PADRE-3C3d vaccine induced significantly higher antibody production in another AD mouse model, 3xTg-AD of the H2(b) haplotype. Finally, this study demonstrated that low concentrations of antibodies generated by both DNA vaccines were not sufficient for the reduction of Aβ pathology in the brains of vaccinated Tg2576 animals, confirming previous reports from preclinical studies and the AN-1792 clinical trials, which concluded that the concentration of anti-Aβ antibodies may be essential for the reduction of AD pathology.
Figures


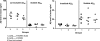
Similar articles
-
Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide.J Immunol. 2005 Feb 1;174(3):1580-6. doi: 10.4049/jimmunol.174.3.1580. J Immunol. 2005. PMID: 15661919
-
Refinement of a DNA based Alzheimer's disease epitope vaccine in rabbits.Hum Vaccin Immunother. 2013 May;9(5):1002-10. doi: 10.4161/hv.23875. Epub 2013 Feb 11. Hum Vaccin Immunother. 2013. PMID: 23399748 Free PMC article.
-
DNA epitope vaccine containing complement component C3d enhances anti-amyloid-beta antibody production and polarizes the immune response towards a Th2 phenotype.J Neuroimmunol. 2008 Dec 15;205(1-2):57-63. doi: 10.1016/j.jneuroim.2008.08.016. Epub 2008 Oct 5. J Neuroimmunol. 2008. PMID: 18838175 Free PMC article.
-
Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials.Neurobiol Dis. 2020 Jun;139:104823. doi: 10.1016/j.nbd.2020.104823. Epub 2020 Feb 28. Neurobiol Dis. 2020. PMID: 32119976 Free PMC article. Review.
-
Abeta DNA vaccination for Alzheimer's disease: focus on disease prevention.CNS Neurol Disord Drug Targets. 2010 Apr;9(2):207-16. doi: 10.2174/187152710791012080. CNS Neurol Disord Drug Targets. 2010. PMID: 20205639 Free PMC article. Review.
Cited by
-
Vaccines for Alzheimer's disease: a brief scoping review.Neurol Sci. 2025 Jul;46(7):2925-2950. doi: 10.1007/s10072-025-08073-2. Epub 2025 Mar 20. Neurol Sci. 2025. PMID: 40111670
-
Immunostimulant patches containing Escherichia coli LT enhance immune responses to DNA- and recombinant protein-based Alzheimer's disease vaccines.J Neuroimmunol. 2014 Mar 15;268(1-2):50-7. doi: 10.1016/j.jneuroim.2014.01.002. Epub 2014 Jan 15. J Neuroimmunol. 2014. PMID: 24507620 Free PMC article.
-
Efficacy and Safety of the Immunization with DNA for Alzheimer's Disease in Animal Models: A Systematic Review from Literature.J Alzheimers Dis Rep. 2017 Dec 2;1(1):195-217. doi: 10.3233/ADR-170025. J Alzheimers Dis Rep. 2017. PMID: 30480238 Free PMC article.
-
Natural IgG antibodies to β amyloid are decreased in patients with Parkinson's disease.Immun Ageing. 2023 Mar 11;20(1):13. doi: 10.1186/s12979-023-00336-w. Immun Ageing. 2023. PMID: 36906630 Free PMC article.
-
Combined treatment of Aβ immunization with statin in a mouse model of Alzheimer's disease.J Neuroimmunol. 2012 Mar;244(1-2):70-83. doi: 10.1016/j.jneuroim.2012.01.008. Epub 2012 Feb 11. J Neuroimmunol. 2012. PMID: 22326143 Free PMC article.
References
-
- Agadjanyan M.G. Ghochikyan A. Petrushina I. Vasilevko V. Movsesyan N. Mkrtichyan M. Saing T. Cribbs D.H. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005;174:1580–1586. - PubMed
-
- Ahearn J.M. Fischer M.B. Croix D. Goerg S. Ma M. Xia J. Zhou X. Howard R.G. Rothstein T.L. Carroll M.C. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. - PubMed
-
- Alexander J. del Guercio M.F. Maewal A. Qiao L. Fikes J. Chesnut R.W. Paulson J. Bundle D.R. DeFrees S. Sette A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000;164:1625–1633. - PubMed
-
- Babiuk S. Baca-Estrada M.E. Foldvari M. Storms M. Rabussay D. Widera G. Babiuk L.A. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. - PubMed
-
- Bard F. Cannon C. Barbour R. Burke R.L. Games D. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Lieberburg I. Motter R. Nguyen M. Soriano F. Vasquez N. Weiss K. Welch B. Seubert P. Schenk D. Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

