Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors
- PMID: 20528675
- PMCID: PMC2965195
- DOI: 10.1089/ten.TEA.2010.0166
Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors
Abstract
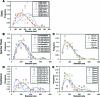
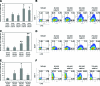
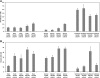
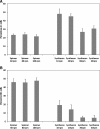
Embryonic stem (ES)-cell-derived lineage-specific stem cells, for example, hematopoietic stem cells, could provide a potentially unlimited source for transplantable cells, especially for cell-based therapies. However, reproducible methods must be developed to maximize and scale-up ES cell differentiation to produce clinically relevant numbers of therapeutic cells. Bioreactor-based dynamic culture conditions are amenable to large-scale cell production, but few studies have evaluated how various bioreactor types and culture parameters influence ES cell differentiation, especially hematopoiesis. Our results indicate that cell seeding density and bioreactor speed significantly affect embryoid body formation and subsequent generation of hematopoietic stem and progenitor cells in both stirred tank (spinner flask) and rotary microgravity (Synthecon™) type bioreactors. In general, high percentages of hematopoietic stem and progenitor cells were generated in both bioreactors, especially at high cell densities. In addition, Synthecon bioreactors produced more sca-1(+) progenitors and spinner flasks generated more c-Kit(+) progenitors, demonstrating their unique differentiation profiles. cDNA microarray analysis of genes involved in pluripotency, germ layer formation, and hematopoietic differentiation showed that on day 7 of differentiation, embryoid bodies from both bioreactors consisted of all three germ layers of embryonic development. However, unique gene expression profiles were observed in the two bioreactors; for example, expression of specific hematopoietic genes were significantly more upregulated in the Synthecon cultures than in spinner flasks. We conclude that bioreactor type and culture parameters can be used to control ES cell differentiation, enhance unique progenitor cell populations, and provide means for large-scale production of transplantable therapeutic cells.
Figures




References
-
- Sato N. Sanjuan I.M. Heke M. Uchida M. Naef F. Brivanlou A.H. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404. - PubMed
-
- Kaji E.H. Leiden J.M. Gene and stem cell therapies. JAMA. 2001;285:545. - PubMed
-
- Storb R. Allogeneic hematopoietic stem cell transplantation—yesterday, today, and tomorrow. Exp Hematol. 2003;31:1. - PubMed
-
- Storb R. McSweeney P.A. Sandmaier B.M. Nash R.A. Georges G. Maloney D.G., et al. Allogeneic hematopoietic stem cell transplantation: from the nuclear age into the twenty-first century. Transplant Proc. 2000;32:2548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

